Introduction
Both products can be used to clean any greasy surface, although it's best to choose one over the other depending on the surface.
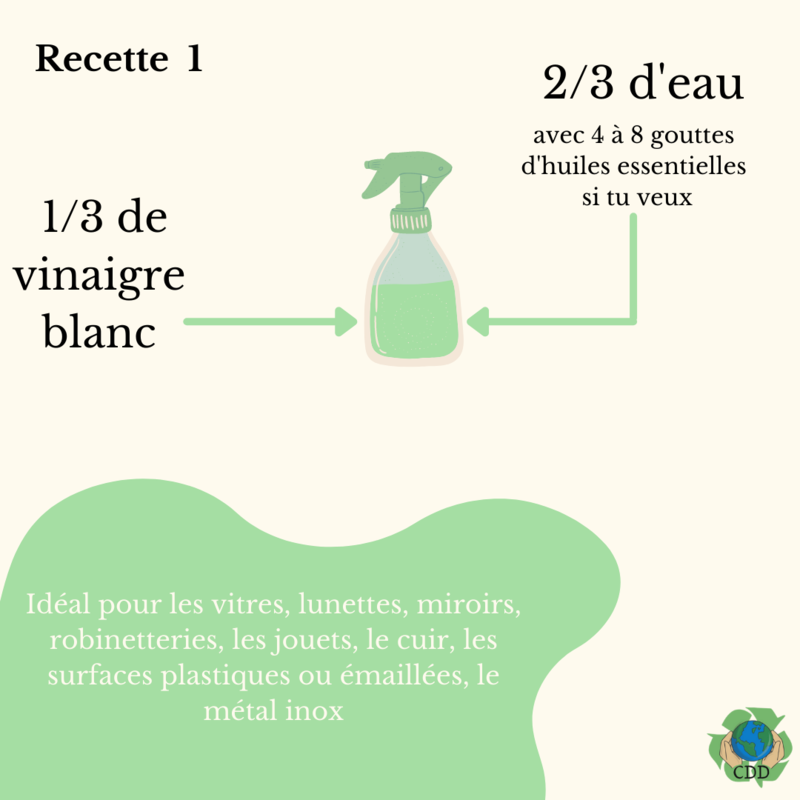
- Vinegar cleaner: for windows, glasses, mirrors, taps, baby/children's toys and leather.
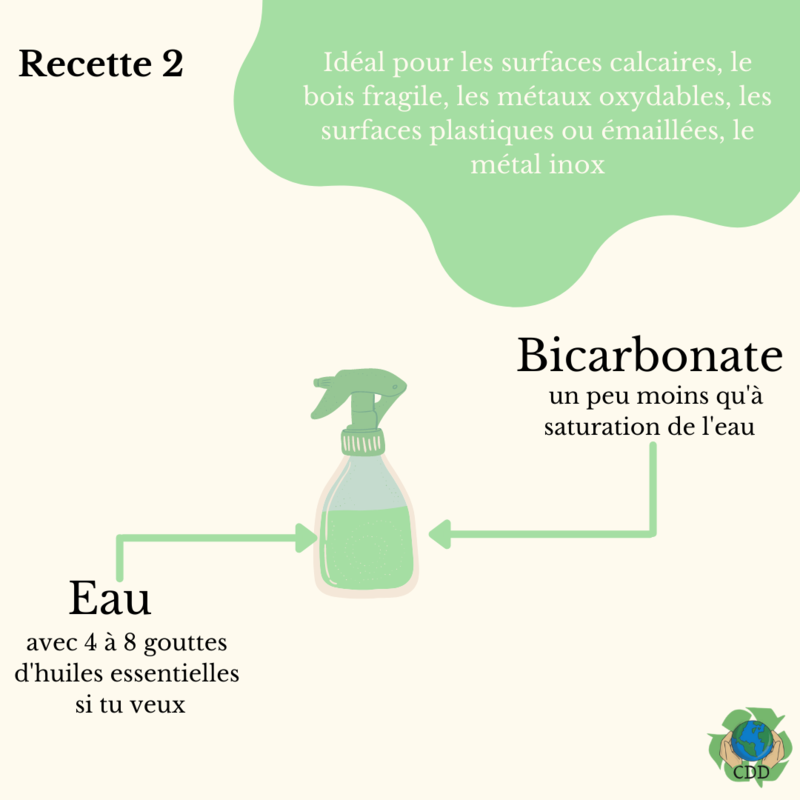
- Bicarbonate cleaner: for hard surfaces, fragile wood, oxidisable metals, etc.
- One or the other: for plastic, enamel and stainless steel surfaces.
Matériaux
- White vinegar
- Sodium bicarbonate
- Water
Outils
empty sprinkler bottles or conventional bottles
Étape 1 - Details of ingredients
- White vinegar: White vinegar, otherwise known as spirit vinegar, crystal. It is a 100% natural and biodegradable product with a pH between 3.5 and 5 (acid) with approximately 12% alcohol, obtained by macerating an aqueous solution of at least 6% acetic acid (often from beet ethanol). It evaporates naturally without posing any risk (apart from a slight and temporary irritation of the throat and eyes if you are in a small space). Cost : ~ 0.40€/L, prefer to buy in the food vinegar section (avoid the household section as it is often more expensive and flavoured). Properties: More info and G culture here
- Degreaser,
- Deodorising,
- Conservative (thanks to the acidic pH),
- Antiseptic, disinfectant, antiparasitic, antibacterial, antifungal (thanks to the acid pH)
- Descaler / anti-scale (the acid reacts with the scale to make it disappear in an acid-base reaction causing heat and CO2 bubbles),
- Corrosive for limestone surfaces (and damages wood in high doses).
- Sodium bicarbonate: also known as sodium bicarbonate, sodium hydrogen carbonate, sodium hydrogen carbonate, sodium hydrogen carbonate, monosodium carbonate, sodium bicarbonate, soda, baking soda or natrum bicarbonatum. A fine white powder similar to table salt or talc with a pH of 8-8.5 (base). Made from limestone and salt, bicarbonate is naturally present in the body and is harmless to health. Does not give off any fumes, does not evaporate. Properties: cleans, scours and deodorises. It dissolves grease and proteins responsible for stains. It prevents the proliferation of bacteria and softens hard water. More info and G culture here Cost : ~3-4 euros/kg
Here we'll be using household bicarbonate (cheaper than baking soda, but if you only have baking soda for food or cosmetics on hand it works very well).
.
Étape 2 - Vinegar multi-purpose cleaner
Mix 1/3 volume of white vinegar with 2/3 volume of water.
Optional: to add fragrance, you can replace some of the water with hydrosols OR add 4-8 drops of essential oils per 1 litre.
Étape 3 - Bicarbonate multi-purpose cleaner
Mix bicarbonate and cold water until the water is saturated, then add a little more water. It's important to add more water, otherwise the saturated liquid will recrystallise in the nozzle and end up clogging it.
Optional: to add fragrance, you can replace some of the water with hydrosols OR add 4-8 drops of essential oils per 1 litre.
Notes et références
In my experience, I have a slight preference for the vinegar-based cleaner because it seems to dissolve grease more quickly. It's incredibly effective and has absolutely nothing to envy from industrial products, while being virtually free.
Contrary to what is commonly found on the internet and even in some recipe books, there is no point in mixing vinegar and bicarbonate in the same product unless you want to use it as a descaler at the time of the reaction. Once the gas-producing reaction is over, the cleaning power of the liquid is equivalent to that of water!
Thanks to the student team of the CDD - Comission Développement Durable ISTOM for the illustrations
.
Published

 Français
Français English
English Deutsch
Deutsch Español
Español Italiano
Italiano Português
Português