| (49 révisions intermédiaires par 2 utilisateurs non affichées) | |||
| Ligne 10 : | Ligne 10 : | ||
|Cost=40 | |Cost=40 | ||
|Currency=EUR (€) | |Currency=EUR (€) | ||
| − | |Tags=Biodiesel, Huile de friture usagée, Méthanol, Carburant, Transport, Diesel, Huile, NomadeDesMers | + | |Tags=Biodiesel, Huile de friture usagée, Méthanol, Carburant, Transport, Diesel, Huile, NomadeDesMers, oil |
|SourceLanguage=fr | |SourceLanguage=fr | ||
|Language=en | |Language=en | ||
| Ligne 22 : | Ligne 22 : | ||
"Biodiesel has man benefits, making it an interesting fuel alternative:" | "Biodiesel has man benefits, making it an interesting fuel alternative:" | ||
| − | *It is simple to make yourself. *It can be produced at a low cost* It can be used in any conventional diesel engine. It also allows for better lubrication of the engine. *It contributes to the recycling of organic waste, such as used cooking oil that is widely used in restaurants. *It is made from vegetable oil and therefore releases only a small additional amount of CO2 into the atmosphere. It also reduces the emissions of certain harmful compounds compared to petro-diesel (carbon monoxide, sulphur dioxide, etc) | + | *It is simple to make yourself. |
| + | *It can be produced at a low cost | ||
| + | * It can be used in any conventional diesel engine. It also allows for better lubrication of the engine. | ||
| + | *It contributes to the recycling of organic waste, such as used cooking oil that is widely used in restaurants. | ||
| + | *It is made from vegetable oil and therefore releases only a small additional amount of CO2 into the atmosphere. It also reduces the emissions of certain harmful compounds compared to petro-diesel (carbon monoxide, sulphur dioxide, etc) | ||
| − | Safety measures | + | {{Warning|IMPORTANT : Safety measures |
- Wear safety glasses, a gown, resistant gloves and long clothes. Working with a breathing mask is also recommended. | - Wear safety glasses, a gown, resistant gloves and long clothes. Working with a breathing mask is also recommended. | ||
| Ligne 59 : | Ligne 63 : | ||
|Material='''1.1 Oil''' | |Material='''1.1 Oil''' | ||
| − | Our approach being the most ecological possible, the oil used for making biodiesel is | + | Our approach being the most ecological possible, the oil used for making biodiesel is '''used cooking oil''' collected from all sorts of eating establishments. In order to collect the best sort of oil, talk to the owner of the restaurant as well as the chef so you can explain the purpose of your approach. Collecting oil without permission can be qualified as an offence. Furthermore, make sure you check the quality of the oil collected. Used oil contains free fatty acids. They appear when oil is left in the open air over a certain period of time and becomes rancid or when heated in the presence of water. Frying food (that contains water) therefore causes FFAs to appear in oil. During transesterification, these FFAs react with the catalyst (strong base) and create an unwanted soap. The soap stops the products of the reaction, biodiesel and glycerol, from separating correctly, which creates an emulsion that is difficult to work with. |
The more the cooking oil is used, the more the concentration in FFAs increases and becomes browner. '''Therefore, try to avoid collecting old, brown oil'''. The better the quality of the oil is, the easier the converting process will be. | The more the cooking oil is used, the more the concentration in FFAs increases and becomes browner. '''Therefore, try to avoid collecting old, brown oil'''. The better the quality of the oil is, the easier the converting process will be. | ||
| Ligne 67 : | Ligne 71 : | ||
'''Avoid peanut, coconut and palm oil or animal fats''' because the temperatures at which they solidify are too high (>10°C), even if biodiesel has a lower freezing point than the oil it originates from. Also ban olive oil that is too acidic, these acids could interfere during the reaction in the creating of biodiesel. | '''Avoid peanut, coconut and palm oil or animal fats''' because the temperatures at which they solidify are too high (>10°C), even if biodiesel has a lower freezing point than the oil it originates from. Also ban olive oil that is too acidic, these acids could interfere during the reaction in the creating of biodiesel. | ||
| − | ''1.2 Methanol'' | + | '''1.2 Methanol''' |
Methanol, or wood alcohol, used to be obtained by wood pyrolysis. Nowadays, it is synthesized from natural gas and is mainly used as an antifreeze for coolants, as a solvent for the synthesis of other chemicals or as fuel in the racing field (dragsters, model engines). It can be purchased through suppliers who specialize in chemicals, some DIY stores and garages. | Methanol, or wood alcohol, used to be obtained by wood pyrolysis. Nowadays, it is synthesized from natural gas and is mainly used as an antifreeze for coolants, as a solvent for the synthesis of other chemicals or as fuel in the racing field (dragsters, model engines). It can be purchased through suppliers who specialize in chemicals, some DIY stores and garages. | ||
| Ligne 75 : | Ligne 79 : | ||
{{Warning|It's very flammable and the slightest spark could cause burns or an explosion. It is also toxic and can cause blindness if inhaled or ingested.}} | {{Warning|It's very flammable and the slightest spark could cause burns or an explosion. It is also toxic and can cause blindness if inhaled or ingested.}} | ||
| − | ''1.3 Catalyst'' | + | '''1.3 Catalyst''' |
Either sodium hydroxide (NaOH, caustic soda) or potassium hydroxide (KOH, caustic potash) can be used as a catalyst. They are both corrosive and highly basic. These chemicals are quite common and can be found in DIY stores, on line or through chemical suppliers. KOH and NaOH are both hygroscopic, which means they absorb the humidity in the atmosphere quickly. They become less efficient catalysts in the presence of water: always keep them in sealed, airtight containers. | Either sodium hydroxide (NaOH, caustic soda) or potassium hydroxide (KOH, caustic potash) can be used as a catalyst. They are both corrosive and highly basic. These chemicals are quite common and can be found in DIY stores, on line or through chemical suppliers. KOH and NaOH are both hygroscopic, which means they absorb the humidity in the atmosphere quickly. They become less efficient catalysts in the presence of water: always keep them in sealed, airtight containers. | ||
| − | ''NaOH vs KOH, different properties. | + | '''NaOH vs KOH, different properties.''' |
* Sodium hydroxide (NaOH) is less expensive and commonly used to make biodiesel on a small scale. However, sodium hydroxide (NaOH) contaminates the water used for cleaning biodiesel and must not be disposed of in nature. We therefore suggest you don't use it if you cannot ensure water treatment. | * Sodium hydroxide (NaOH) is less expensive and commonly used to make biodiesel on a small scale. However, sodium hydroxide (NaOH) contaminates the water used for cleaning biodiesel and must not be disposed of in nature. We therefore suggest you don't use it if you cannot ensure water treatment. | ||
* Potassium hydroxide (KOH) having higher catalysis properties, is gaining in popularity. Potassium hydroxide (KOH) dissolves easier in methanol and is less sensitive to water. Furthermore, the glycerin obtained through the reaction remains liquid and can be added to compost with more safety or used in small amounts as a supplement for animal feed. | * Potassium hydroxide (KOH) having higher catalysis properties, is gaining in popularity. Potassium hydroxide (KOH) dissolves easier in methanol and is less sensitive to water. Furthermore, the glycerin obtained through the reaction remains liquid and can be added to compost with more safety or used in small amounts as a supplement for animal feed. | ||
| − | |Tools=''Transesterification'' | + | |Tools='''Transesterification''' |
* Used vegetable oil {{For information| For your first attempts, we recommend you try with just a small amount and use new vegetable oil}} | * Used vegetable oil {{For information| For your first attempts, we recommend you try with just a small amount and use new vegetable oil}} | ||
| Ligne 92 : | Ligne 96 : | ||
* 1 clean, dry container with a drain tube or a tap for when you want to carry out the reaction {{for information| we highly recommend using a silo shaped canister such as the one shown on the photo. This will make all the different draining stages a lot easier. Without this system, it can turn out to be quite tricky and time consuming.}} | * 1 clean, dry container with a drain tube or a tap for when you want to carry out the reaction {{for information| we highly recommend using a silo shaped canister such as the one shown on the photo. This will make all the different draining stages a lot easier. Without this system, it can turn out to be quite tricky and time consuming.}} | ||
| − | * | + | * 1 submersible pump so you can do the blending in the canister |
| + | * 1 one hot plate or heating resistor | ||
| + | * 1 temperature sensor | ||
| + | * 1 precision balance | ||
| + | * 1 funnel | ||
| + | * 1 big glass jar shut or a stainless steel tank for the blending of the methanol and the catalyst | ||
| + | * 1 measuring glass for the dosage of methanol | ||
| + | * rubber gloves | ||
| + | * safety glasses | ||
| − | + | '''Filtration''' | |
| − | + | *1 canister of new oil | |
| + | *1 clean and empty oil canister | ||
| + | * 1 funnel | ||
| + | * 1 cotton sheet/ 1 sock | ||
| + | * a 1 to 5µm filter bag | ||
| − | + | '''Titration''' | |
| − | * | + | * 10ml of isopropyl alcohol or isopropanol. |
| + | * 1ml of your used oil | ||
| + | * 2-3 drops of phenolphthalein (indicator) | ||
| + | * 1L [https://fr.wikihow.com/fabriquer-de-l%27eau-distill%C3%A9e of distilled water] | ||
| + | * 1g of catalyst, sodium hydroxide NaOH or potassium hydroxide KOH. | ||
| + | {{Warning| Always keep these products in a sealed, airtight container}} | ||
* - 1 scale* - 1 small container/glass jar * - 3 measuring cylinders/tubes * - Two 1mL graduated syringes or cruets. Please take note that one syringe is for the oil and the other one is for the soda-water blend. * - Rubber gloves * - Safety glasses- Breathing mask | * - 1 scale* - 1 small container/glass jar * - 3 measuring cylinders/tubes * - Two 1mL graduated syringes or cruets. Please take note that one syringe is for the oil and the other one is for the soda-water blend. * - Rubber gloves * - Safety glasses- Breathing mask | ||
| − | + | '''Distillation of glycerol''' | |
| − | * | + | * 1 pressure cooker |
| + | * a 5m copper pipe | ||
| + | * a 50cm heat-resistant flexible tube (silicone, Teflon) | ||
| + | * Conventional pipe clamps for plumbing | ||
}} | }} | ||
{{Tuto Step | {{Tuto Step | ||
| Ligne 116 : | Ligne 140 : | ||
The two first methods look easy, but as for many things, it isn’t that simple. | The two first methods look easy, but as for many things, it isn’t that simple. | ||
| − | ''1. Blends | + | '''1. Blends''' |
Vegetable oil is a lot more viscous (thicker) than petro-diesel or biodiesel The purpose of blending or mixing SVOs with other fuels or solvents is to reduce the viscosity to make it thinner, so it will flow easier through the fuel system to the combustion chamber. However, this is not the only problem when using SVOs. Its chemical properties and combustion characteristics are different to those of petro-diesel, for which diesel engines and their fuel systems are designed for. Diesel engines, especially the most modern ones, are high-tech machines with specific fuel needs (see [http://journeytoforever.org/biodiesel_TDI.html la controverse TDI-SVO]). | Vegetable oil is a lot more viscous (thicker) than petro-diesel or biodiesel The purpose of blending or mixing SVOs with other fuels or solvents is to reduce the viscosity to make it thinner, so it will flow easier through the fuel system to the combustion chamber. However, this is not the only problem when using SVOs. Its chemical properties and combustion characteristics are different to those of petro-diesel, for which diesel engines and their fuel systems are designed for. Diesel engines, especially the most modern ones, are high-tech machines with specific fuel needs (see [http://journeytoforever.org/biodiesel_TDI.html la controverse TDI-SVO]). | ||
| Ligne 132 : | Ligne 156 : | ||
Lastly, blending SVOs with other solvents like mineral turpentine (white spirit) or other "secret" ingredients such as naphthalene or xylol or with unleaded petrol are only experimental processes at this point in time and the effects of these additives on the characteristics of fuel combustion or their long term effects on the engine remain unknown. We highly recommend you don't choose this kind of solution: use such blends at your own risk. | Lastly, blending SVOs with other solvents like mineral turpentine (white spirit) or other "secret" ingredients such as naphthalene or xylol or with unleaded petrol are only experimental processes at this point in time and the effects of these additives on the characteristics of fuel combustion or their long term effects on the engine remain unknown. We highly recommend you don't choose this kind of solution: use such blends at your own risk. | ||
| − | ''2. Straight vegetable oil with engine modification | + | '''2. Straight vegetable oil with engine modification''' |
Direct SVO fuel systems can be a clean, efficient and economical option. | Direct SVO fuel systems can be a clean, efficient and economical option. | ||
| − | Unlike biodiesel, you will need to modify your engine so you can use SVO. The best way to get one is to purchase a [ | + | Unlike biodiesel, you will need to modify your engine so you can use SVO. The best way to get one is to purchase a [http://journeytoforever.org/biodiesel_svo.html#1tank professional single-tank SVO system] with optimized injector nozzles, glow-plugs for vegetable oil as well as a fuel pre-heating system. With this [http://journeytoforever.org/biodiesel_svo.html#Elsbett system Elsbett system], you can use petro-diesel, biodiesel or SVO, with any combination you like. Start up and go, turn off and stop, just like any other car. |
There are other SVO systems with two tanks that preheat the oil to make it less viscous. You must start your engine up with ordinary petro-diesel ( or biodiesel) in the tank, then switch to SVO in the other tank once the vegetable oil is hot enough (so quite liquid) then come back to petro-diesel before turning the engine off, or you'll clog the injectors. | There are other SVO systems with two tanks that preheat the oil to make it less viscous. You must start your engine up with ordinary petro-diesel ( or biodiesel) in the tank, then switch to SVO in the other tank once the vegetable oil is hot enough (so quite liquid) then come back to petro-diesel before turning the engine off, or you'll clog the injectors. | ||
| Ligne 142 : | Ligne 166 : | ||
You can find lots of information about SVO systems on [http://journeytoforever.org/biodiesel_svo.html here]. | You can find lots of information about SVO systems on [http://journeytoforever.org/biodiesel_svo.html here]. | ||
| − | ''3 Biodiesel or SVO? | + | '''3 Biodiesel or SVO?''' |
Biodiesel has many obvious advantages over SVO: | Biodiesel has many obvious advantages over SVO: | ||
| Ligne 158 : | Ligne 182 : | ||
<u>''On the table opposite is a summary of the main differences between making biodiesel and modifying your engine to use SVO directly. SVO/WVO are the English-speaking equivalences of HVC/HVR in French.''</u> | <u>''On the table opposite is a summary of the main differences between making biodiesel and modifying your engine to use SVO directly. SVO/WVO are the English-speaking equivalences of HVC/HVR in French.''</u> | ||
| − | ''$ Financial considerations: $'' | + | '''$ Financial considerations: $''' |
On average, a French person consumes: 1,3[€/L]*4/100[L/km]*19000[km/an] = 988 €/year, representing roughly 1000€ per year on diesel. | On average, a French person consumes: 1,3[€/L]*4/100[L/km]*19000[km/an] = 988 €/year, representing roughly 1000€ per year on diesel. | ||
| Ligne 173 : | Ligne 197 : | ||
}} | }} | ||
{{Tuto Step | {{Tuto Step | ||
| − | |Step_Title=Oil titration (for used oil only) | + | |Step_Title=Oil titration (for used oil only) |
| − | |Step_Content= | + | |Step_Content=* Weigh precisely 1g of catalyst and dissolve it in 1L of distilled water. |
{{Warning| Always add the catalyst to water. Never pour water on to the catalyst.}} | {{Warning| Always add the catalyst to water. Never pour water on to the catalyst.}} | ||
| − | + | * In a glass measuring container, measure 10mL of isopropanol. | |
| − | + | *Take 1mL of your testing oil with a graduated syringe and add it to the isopropanol. | |
| − | + | * Add 3 drops of phenolphthalein to the blend. | |
| − | + | * Stir the blend thoroughly, with a magnetic stirrer if possible. | |
| − | + | * Fill the graduated cruet up with 10mL of the distilled water/catalyst blend, make sure you rinse the cruet with distilled water before using. | |
| − | + | * Add the distilled water-catalyst to the blend, drop by drop, while stirring continuously. | |
| − | + | * Continue to gradually add the titrant until the end point is obtained. The blend becomes a dark pink colour. The colour change should last 20 seconds. | |
| − | + | * Take note of the exact volume of distilled water/catalyst (V<sub>titration</sub>) used to neutralize the acidity of the oil. | |
| − | + | * Do a second test and calculate the average of your tests for more precision. | |
| − | + | * Use this information to find out the amount of catalyst you need to add to the reaction following this formula: (To understand more about the formula, see ANNEX 2 of [http://carburerauxalgues.com/site/download/download_file_st/ActiviteI_ELEVES/pdf this document]) | |
| − | |||
| − | |||
| − | + | '''Catalyst mass required (g) = (B+ V<sub>titration</sub> (mL)) x Volume of oil (L)''' | |
With: | With: | ||
| Ligne 224 : | Ligne 246 : | ||
|Step_Content=- Heat the oil at 60°C and maintain at this temperature. | |Step_Content=- Heat the oil at 60°C and maintain at this temperature. | ||
| − | + | {{Warning| Make sure you don't exceed this temperature! The boiling point of methanol is 65°C and overpressure could occur due to the production of methanol vapour in the closed container.}} | |
| − | {{Warning| Make sure you don't exceed this temperature! The | ||
| − | |||
- Pour the methanol into the glass container. | - Pour the methanol into the glass container. | ||
| Ligne 238 : | Ligne 258 : | ||
- Stir and let the catalyst dissolve completely in the methanol. This takes roughly 2 minutes. And once again, be careful: the blend deteriorates quickly. Carry on with the next stage as soon as the catalyst/methanol blend is homogeneous. | - Stir and let the catalyst dissolve completely in the methanol. This takes roughly 2 minutes. And once again, be careful: the blend deteriorates quickly. Carry on with the next stage as soon as the catalyst/methanol blend is homogeneous. | ||
| − | |||
- Add the catalyst/methanol blend to the hot oil (60°C). Leave the elements to blend together for several hours. Two products are formed from this reaction; biodiesel and glycerol. Biodiesel being less dense, it forms the top layer. | - Add the catalyst/methanol blend to the hot oil (60°C). Leave the elements to blend together for several hours. Two products are formed from this reaction; biodiesel and glycerol. Biodiesel being less dense, it forms the top layer. | ||
| − | |||
-Wait 24 hours or more before carrying out the next stage. | -Wait 24 hours or more before carrying out the next stage. | ||
| Ligne 257 : | Ligne 275 : | ||
|Step_Content=-Slowly add the washing solution representing the equivalent of 20% the volume of biodiesel, to avoid the formation of soap and wait 10 minutes. Drain off the washing water. | |Step_Content=-Slowly add the washing solution representing the equivalent of 20% the volume of biodiesel, to avoid the formation of soap and wait 10 minutes. Drain off the washing water. | ||
| − | |||
{{Ideal| Using a spray to obtain fine droplets could be an interesting solution.}} | {{Ideal| Using a spray to obtain fine droplets could be an interesting solution.}} | ||
| − | |||
-Carry on with a second wash. This time, slowly turn the container upside down 4 to 5 times and wait 10 minutes. Excessive agitation could result in the formation of an emulsion and delay the process. Drain off the washing water | -Carry on with a second wash. This time, slowly turn the container upside down 4 to 5 times and wait 10 minutes. Excessive agitation could result in the formation of an emulsion and delay the process. Drain off the washing water | ||
| Ligne 273 : | Ligne 289 : | ||
{{Tuto Step | {{Tuto Step | ||
|Step_Title=Treating glycerol | |Step_Title=Treating glycerol | ||
| − | |Step_Content= | + | |Step_Content={{Warning|: This stage, although it's essential, must be carried out with extreme precaution. Methanol is an extremely flammable and explosive gas. This operation is to be carried out in a well-ventilated area. Avoid open flame heating as much as possible. Be careful when choosing your still, the simultaneous increase in pressure and temperature could make the blend explosive. }} Recovery of methanol can be obtained by the distillation of glycerol, by heating it at a temperature above 65% which is the boiling point of methanol. Methanol vapours then go through a condenser, are cooled down and collected in liquid form from the outlet. |
| − | {{Warning|: This stage, although it's essential, must be carried out with extreme precaution. Methanol is an extremely flammable and explosive gas. This operation is to be carried out in a well-ventilated area. Avoid open flame heating as much as possible. Be careful when choosing your still, the simultaneous increase in pressure and temperature could make the blend explosive. }} Recovery of methanol can be obtained by the distillation of glycerol, by heating it at a temperature above 65% which is the boiling point of methanol. Methanol vapours then go through a condenser, are cooled down and collected in liquid form from the outlet. | ||
| − | |||
| − | Video tutorial on making your own still: https://www.youtube.com/watch?v=5H1UVv8FaO8 | + | '''Video tutorial on making your own still: https://www.youtube.com/watch?v=5H1UVv8FaO8 ''' |
• Form a coil with the copper pipe (around a canister for example). Leave both ends straight, roughly 30cm long. | • Form a coil with the copper pipe (around a canister for example). Leave both ends straight, roughly 30cm long. | ||
Version actuelle datée du 8 juin 2023 à 15:10
Description
This tutorial gives you the process on how to make biodiesel on a medium-scale (about 50L) from used cooking oil. This tutorial was written following the Nomade des Mers crew's visit to Cambodia. The social enterprise Naga Earth collects and converts used cooking oil from the town's hotels and restaurants into biodiesel. They also make soap with glycerin that comes from this same reaction. This benefits various schools and associations who carry out hygiene programs throughout the region.
Sommaire
Sommaire
- 1 Description
- 2 Sommaire
- 3 Introduction
- 4 Video d'introduction
- 5 Étape 1 - Identify your needs
- 6 Étape 2 - Filtration
- 7 Étape 3 - Oil titration (for used oil only)
- 8 Étape 4 - Transesterification
- 9 Étape 5 - Separating the glycerol.
- 10 Étape 6 - The washing and drying of biodiesel.
- 11 Étape 7 - Treating glycerol
- 12 Notes et références
- 13 Commentaires
Introduction
Biodiesel is an alternative fuel to petro-sourced diesel. It can be used alone in engines or blended with petro diesel with different concentration levels. This fuel is obtained from vegetable oil or animal fat that is converted by a chemical process named "transesterification". It involves making oil react with an alcohol (methanol or ethanol) and a catalyst (sodium or potassium hydroxide) in order to obtain methyl or ethyl esters (biodiesel) and a by-product called glycerin.
Biodiesel can be made in various amounts. The processes described here are suitable for occasional production and small amounts. Because the process requires practice, we recommend you start by making small amounts then gradually go towards a larger scale of production.
"Biodiesel has man benefits, making it an interesting fuel alternative:"
- It is simple to make yourself.
- It can be produced at a low cost
- It can be used in any conventional diesel engine. It also allows for better lubrication of the engine.
- It contributes to the recycling of organic waste, such as used cooking oil that is widely used in restaurants.
- It is made from vegetable oil and therefore releases only a small additional amount of CO2 into the atmosphere. It also reduces the emissions of certain harmful compounds compared to petro-diesel (carbon monoxide, sulphur dioxide, etc)
If you wish to reduce both your consumption and expenses on fossil fuel, there are several options available:
- vegetable oil fuel blended with diesel
- Vegetable oil fuel with engine modification
Youtube
Matériaux
1.1 Oil
Our approach being the most ecological possible, the oil used for making biodiesel is used cooking oil collected from all sorts of eating establishments. In order to collect the best sort of oil, talk to the owner of the restaurant as well as the chef so you can explain the purpose of your approach. Collecting oil without permission can be qualified as an offence. Furthermore, make sure you check the quality of the oil collected. Used oil contains free fatty acids. They appear when oil is left in the open air over a certain period of time and becomes rancid or when heated in the presence of water. Frying food (that contains water) therefore causes FFAs to appear in oil. During transesterification, these FFAs react with the catalyst (strong base) and create an unwanted soap. The soap stops the products of the reaction, biodiesel and glycerol, from separating correctly, which creates an emulsion that is difficult to work with.
The more the cooking oil is used, the more the concentration in FFAs increases and becomes browner. Therefore, try to avoid collecting old, brown oil. The better the quality of the oil is, the easier the converting process will be.
When making biodiesel, prefer using oil such as vegetable oil with a neutral pH or nearly neutral such as rapeseed, corn oil or sunflower oil. These oils have a low freezing point, which means they don't solidify if temperatures drop in winter (freezing temperatures for these oils range from -10°C to -2°C for rapeseed, -15°C for sunflower).
Avoid peanut, coconut and palm oil or animal fats because the temperatures at which they solidify are too high (>10°C), even if biodiesel has a lower freezing point than the oil it originates from. Also ban olive oil that is too acidic, these acids could interfere during the reaction in the creating of biodiesel.
1.2 Methanol
Methanol, or wood alcohol, used to be obtained by wood pyrolysis. Nowadays, it is synthesized from natural gas and is mainly used as an antifreeze for coolants, as a solvent for the synthesis of other chemicals or as fuel in the racing field (dragsters, model engines). It can be purchased through suppliers who specialize in chemicals, some DIY stores and garages.
1.3 Catalyst
Either sodium hydroxide (NaOH, caustic soda) or potassium hydroxide (KOH, caustic potash) can be used as a catalyst. They are both corrosive and highly basic. These chemicals are quite common and can be found in DIY stores, on line or through chemical suppliers. KOH and NaOH are both hygroscopic, which means they absorb the humidity in the atmosphere quickly. They become less efficient catalysts in the presence of water: always keep them in sealed, airtight containers.
NaOH vs KOH, different properties.
- Sodium hydroxide (NaOH) is less expensive and commonly used to make biodiesel on a small scale. However, sodium hydroxide (NaOH) contaminates the water used for cleaning biodiesel and must not be disposed of in nature. We therefore suggest you don't use it if you cannot ensure water treatment.
- Potassium hydroxide (KOH) having higher catalysis properties, is gaining in popularity. Potassium hydroxide (KOH) dissolves easier in methanol and is less sensitive to water. Furthermore, the glycerin obtained through the reaction remains liquid and can be added to compost with more safety or used in small amounts as a supplement for animal feed.
Outils
Transesterification
- Used vegetable oil Modèle:For information
- 250 mL of methanol/ litre of oil * If you are using new oil : 5,5g of NaOH or 7g of KOH / litre of oil. *If you are using used oil: Calculate the catalyst mass that must be added when you get to the titration stage. Modèle:For information
- 1 clean, dry container with a drain tube or a tap for when you want to carry out the reaction Modèle:For information
- 1 submersible pump so you can do the blending in the canister
- 1 one hot plate or heating resistor
- 1 temperature sensor
- 1 precision balance
- 1 funnel
- 1 big glass jar shut or a stainless steel tank for the blending of the methanol and the catalyst
- 1 measuring glass for the dosage of methanol
- rubber gloves
- safety glasses
Filtration
- 1 canister of new oil
- 1 clean and empty oil canister
- 1 funnel
- 1 cotton sheet/ 1 sock
- a 1 to 5µm filter bag
Titration
- 10ml of isopropyl alcohol or isopropanol.
- 1ml of your used oil
- 2-3 drops of phenolphthalein (indicator)
- 1L of distilled water
- 1g of catalyst, sodium hydroxide NaOH or potassium hydroxide KOH.
- - 1 scale* - 1 small container/glass jar * - 3 measuring cylinders/tubes * - Two 1mL graduated syringes or cruets. Please take note that one syringe is for the oil and the other one is for the soda-water blend. * - Rubber gloves * - Safety glasses- Breathing mask
Distillation of glycerol
- 1 pressure cooker
- a 5m copper pipe
- a 50cm heat-resistant flexible tube (silicone, Teflon)
- Conventional pipe clamps for plumbing
Étape 1 - Identify your needs
There are at least three ways of making a diesel engine run with vegetable oil:
- blending it with petro-diesel, a solvent or petrol; *using it as it is - usually called huile végétale carburant (HVC, SVO in English) or recycled vegetable oil (HVR, WVO in English); *converting it into biodiesel.
The two first methods look easy, but as for many things, it isn’t that simple.
1. Blends
Vegetable oil is a lot more viscous (thicker) than petro-diesel or biodiesel The purpose of blending or mixing SVOs with other fuels or solvents is to reduce the viscosity to make it thinner, so it will flow easier through the fuel system to the combustion chamber. However, this is not the only problem when using SVOs. Its chemical properties and combustion characteristics are different to those of petro-diesel, for which diesel engines and their fuel systems are designed for. Diesel engines, especially the most modern ones, are high-tech machines with specific fuel needs (see la controverse TDI-SVO).
People use various blends, ranging from 10% SVO and 90% petro-diesel to 90% SVO and 10% petro-diesel. Some people use it as it is, without preheating (which makes the vegetable oil a lot less viscous). Some even use 100% SVO without preheating. They often don't realize the long term effects on the engine
You might manage it during the summer period with something like an old 5 cylinder Mercedes-Benz that's over 80 years old, that has a very resistant and tolerant engine. It won't like it but you probably won't destroy the engine. Otherwise, it probably isn't a good idea.
I can't guarantee it but using a blend containing up to 20% of good quality vegetable oil with 80% of petro-diesel is known to be quite safe for old diesels, especially in summer.
If you blend SVO with petro-diesel, you'll still be using fossil fuels - so you'll be cleaner than average, but still not enough for others. Yet, for each litre of SVO you use, you save one litre of fossil fuel and lower carbon dioxide emissions. dioxyde de carbone
However, in order to do it correctly and safely all year round, you will need a system suitable for SVO, including a preheating fuel system at the least (see below) and in that case, blending isn't needed, you can simply use 100% SVO.
Lastly, blending SVOs with other solvents like mineral turpentine (white spirit) or other "secret" ingredients such as naphthalene or xylol or with unleaded petrol are only experimental processes at this point in time and the effects of these additives on the characteristics of fuel combustion or their long term effects on the engine remain unknown. We highly recommend you don't choose this kind of solution: use such blends at your own risk.
2. Straight vegetable oil with engine modification
Direct SVO fuel systems can be a clean, efficient and economical option.
Unlike biodiesel, you will need to modify your engine so you can use SVO. The best way to get one is to purchase a professional single-tank SVO system with optimized injector nozzles, glow-plugs for vegetable oil as well as a fuel pre-heating system. With this system Elsbett system, you can use petro-diesel, biodiesel or SVO, with any combination you like. Start up and go, turn off and stop, just like any other car.
There are other SVO systems with two tanks that preheat the oil to make it less viscous. You must start your engine up with ordinary petro-diesel ( or biodiesel) in the tank, then switch to SVO in the other tank once the vegetable oil is hot enough (so quite liquid) then come back to petro-diesel before turning the engine off, or you'll clog the injectors.
You can find lots of information about SVO systems on here.
3 Biodiesel or SVO?
Biodiesel has many obvious advantages over SVO:
- It works in any diesel car, without any conversion or modification of the engine or fuel system - all you need to do is put it in the tank and start your car up. *It also has better properties than SVO when the weather is cold (but not as good as those of petro-diesel-see here for the use of biodiesel in winter). *Unlike SVO, many tests have been carried out on the long term in many countries, covering millions of kilometres.
Biodiesel is a clean replacement fuel, safe and ready to use, whereas many SVO systems are still experimental and need to be developed.
On the other hand, biodiesel can be more expensive, depending on the amount you produce, what you get from it and the comparison with oil or new petrol. And unlike SVO, it requires treatment. (The purpose of this tutorial). But the community of biodiesel producers that is continuously expanding, doesn't seem too concerned about the matter - they do their weekly or monthly produce and become familiar with the process quite quickly. Many have been doing so for many years.
Anyhow, SVO must also be treated, especially WVO that many people use with SVO systems because they are cheap and sometimes even free. When using WVO, food particles, impurities and water must be removed from it. It will also need to be deacidified. Biodiesel producers think: "If I need to do all that, I might as well make biodiesel instead". But SVO supporters will claim it requires a lot less treatment than biodiesel.
Everyone's taste is different....the decision is yours!
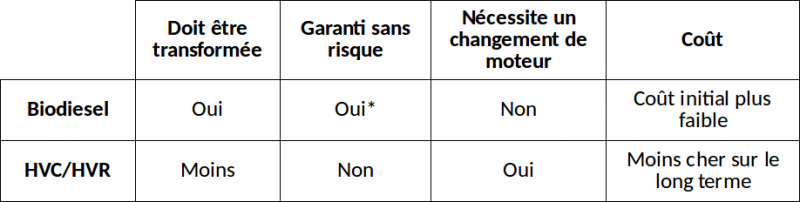
On the table opposite is a summary of the main differences between making biodiesel and modifying your engine to use SVO directly. SVO/WVO are the English-speaking equivalences of HVC/HVR in French.
$ Financial considerations: $
On average, a French person consumes: 1,3[€/L]*4/100[L/km]*19000[km/an] = 988 €/year, representing roughly 1000€ per year on diesel.
Biodiesel costs between 0.5€ and 1€ per litre to produce from recycled oil, representing between 380 and 760€ per year. A good treatment system, adapted to this production volume can be built for roughly 100€. The cost is therefore recovered within a few months.
A SVO system costs between 900 and 1800€. If SVO is free, the cost will therefore be recovered within 1 to 2 years.
Étape 2 - Filtration
Before it can be converted, the used oil must be filtered first in order to remove any possible food residues (fries, doughnuts...). We recommend you let the oil settle for a few days. An initial filtration must be carried out to get rid of the biggest particles (e.g.: with a double cotton sheet or a sock...). Then pour the oil into a 5µm maximum filter bag (the finer the better) above the clean canister, prepared beforehand.
Étape 3 - Oil titration (for used oil only)
- Weigh precisely 1g of catalyst and dissolve it in 1L of distilled water.
- In a glass measuring container, measure 10mL of isopropanol.
- Take 1mL of your testing oil with a graduated syringe and add it to the isopropanol.
- Add 3 drops of phenolphthalein to the blend.
- Stir the blend thoroughly, with a magnetic stirrer if possible.
- Fill the graduated cruet up with 10mL of the distilled water/catalyst blend, make sure you rinse the cruet with distilled water before using.
- Add the distilled water-catalyst to the blend, drop by drop, while stirring continuously.
- Continue to gradually add the titrant until the end point is obtained. The blend becomes a dark pink colour. The colour change should last 20 seconds.
- Take note of the exact volume of distilled water/catalyst (Vtitration) used to neutralize the acidity of the oil.
- Do a second test and calculate the average of your tests for more precision.
- Use this information to find out the amount of catalyst you need to add to the reaction following this formula: (To understand more about the formula, see ANNEX 2 of this document)
Catalyst mass required (g) = (B+ Vtitration (mL)) x Volume of oil (L)
With:
Vtitration: volume of titrant added to neutralize the acidity of the oil (mL)
B: base quantity (catalyst) required for the reaction with unused oil.
B (KOH) = 7.0 g/L
B (NaOH) = 5.5 g/L
Étape 4 - Transesterification
- Heat the oil at 60°C and maintain at this temperature.
- Pour the methanol into the glass container.
- Weigh the amount of soda calculated during the previous stage if using used oil and add to the methanol.
- Stir and let the catalyst dissolve completely in the methanol. This takes roughly 2 minutes. And once again, be careful: the blend deteriorates quickly. Carry on with the next stage as soon as the catalyst/methanol blend is homogeneous.
- Add the catalyst/methanol blend to the hot oil (60°C). Leave the elements to blend together for several hours. Two products are formed from this reaction; biodiesel and glycerol. Biodiesel being less dense, it forms the top layer.
-Wait 24 hours or more before carrying out the next stage.
Étape 5 - Separating the glycerol.
Drain off the glycerol from the bottom of the tank into a new container. Make sure there is no glycerin left in the biodiesel. Keep the glycerol aside.
Étape 6 - The washing and drying of biodiesel.
-Slowly add the washing solution representing the equivalent of 20% the volume of biodiesel, to avoid the formation of soap and wait 10 minutes. Drain off the washing water.
-Carry on with a second wash. This time, slowly turn the container upside down 4 to 5 times and wait 10 minutes. Excessive agitation could result in the formation of an emulsion and delay the process. Drain off the washing water
- Carry on with a third wash. This time, shake the container energetically for a few minutes. Leave to settle then drain off the washing water.
- Transfer the new, washed biodiesel to a new container
- Heat up to 50°C for a few hours until the biodiesel becomes clear.
Étape 7 - Treating glycerol
Recovery of methanol can be obtained by the distillation of glycerol, by heating it at a temperature above 65% which is the boiling point of methanol. Methanol vapours then go through a condenser, are cooled down and collected in liquid form from the outlet.Video tutorial on making your own still: https://www.youtube.com/watch?v=5H1UVv8FaO8
• Form a coil with the copper pipe (around a canister for example). Leave both ends straight, roughly 30cm long. • Attach the flexible tube to the outlet of the pressure cooker with pipe clamps. • Connect the copper pipe and the flexible tube together with pipe clamps. {For information
Notes et références
https://attra.ncat.org/attra-pub-summaries/?pub=318
http://journeytoforever.org/biodiesel_make.html#start
http://carburerauxalgues.com/site/download/download_file_st/ActiviteI_ELEVES/pdf
http://www.make-biodiesel.org/
Published






















 Français
Français English
English Deutsch
Deutsch Español
Español Italiano
Italiano Português
Português