Description
Batteries are central/the key and expensive elements in stand-alone installations. However, their operation and maintenance are not well known/not well understood by the general public. This tutorial, therefore, has several objectives:
- Present/Explain how a lead-acid battery works.
- Present/Explain the different types of lead acid batteries
- Present/Explain the major causes of degradation of lead batteries.
- Present/Explain the rules for the use and maintenance of lead batteries.
- Introduce the process of desulfation (or regeneration) of lead batteries.
- Present/Explain how a lead-acid battery works.
- Present/Explain the different types of lead acid batteries
- Present/Explain the major causes of degradation of lead batteries.
- Present/Explain the rules for the use and maintenance of lead batteries.
- Introduce the process of desulfation (or regeneration) of lead batteries.
Sommaire
Sommaire
- 1 Description
- 2 Sommaire
- 3 Introduction
- 4 Étape 1 - Composition of a lead-acid battery
- 5 Étape 2 - Operation of a lead acid battery
- 6 Étape 3 - The units of the batteries are indicated as abbreviations which are not always easy to understand. Here is a summary table of the units associated with the batteries:
- 7 Étape 4 - Mechanisms of degradation of Lead Acid Batteries
- 8 Étape 5 - Summary of good practices to adopt with lead acid batteries
- 9 Étape 6 - Desulphation/Regeneration of lead acid batteries
- 10 Notes et références
- 11 Commentaires
Introduction
Batteries are often the most expensive and most fragile constituents of an electrical conversion system. Hence, it is important to take care of them through proper use and monitoring.
Lead acid batteries are very fragile. They are sensitive to overcharging, partial charging, deep discharges, excessively rapid charges, and to temperatures above 20°C. All these factors can lead to premature aging, mainly due to a combination of lack of technical knowledge, poorly- sized systems and erroneous use by a person. If one does not control these factors, the batteries will quickly be damaged.
The damage will result in reduced battery life and, in some cases, there could be irreparable deterioration of batteries. Batteries will last longer when used properly, and so their replacement will be less frequent. In the long run, one can make considerable savings. Another interesting aspect is that the conversion system will be more efficient if the batteries are in a good condition. The better the batteries’ condition, the more efficient the installation will be.
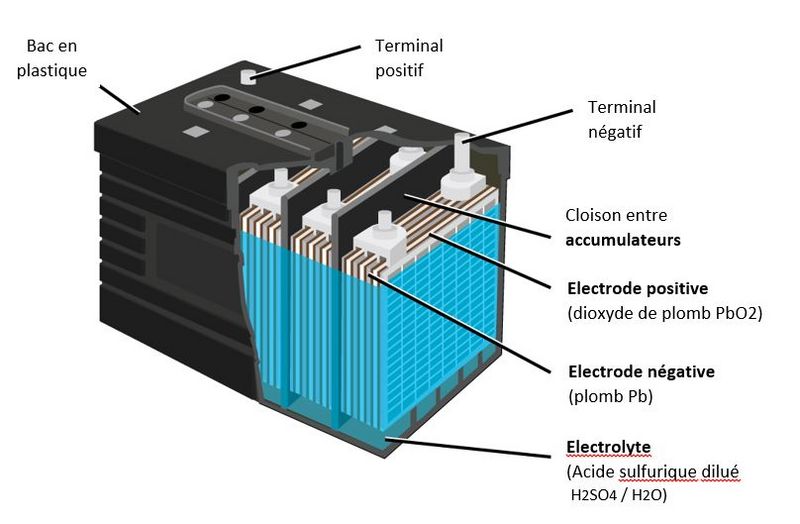
Étape 1 - Composition of a lead-acid battery
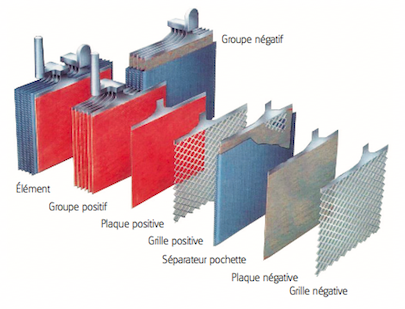
- A lead battery is made up of a set of accumulators./cells. The nominal voltage of an accumulator/cell is approximately 2.1 V, and so a 12-V battery consists of six accumulator/cell mounted in series and connected by welded lead. (A series of cells connected in series, or parallel is called module) The accumulators/cells are fitted/packed in a plastic container and sealed with a lid.
- Each accumulator comprises pairs of positive and negative electrodes (plates) connected in parallel, with a separator in between each pair.
- The separators are generally rectangular sheets, inserted between the positive plates and the negative plates, and have the following important characteristics:
- they serve/act as perfect electrical insulators.
- they are highly permeable to ions carrying electrical charges.
- they have excellent resistance to sulfuric acid,
- The electrodes are composed of a grid on which is deposited a porous active material: lead (Pb) on the negative electrode and lead dioxide (PbO2) on the positive electrode. The grid collects the current and also serves as a mechanical support for the active material.
- The electrolyte is a dilute solution of sulfuric acid in which the electrodes are immersed. It can be in liquid, gel or absorbed form in fiberglass felts, depending on the type of battery.
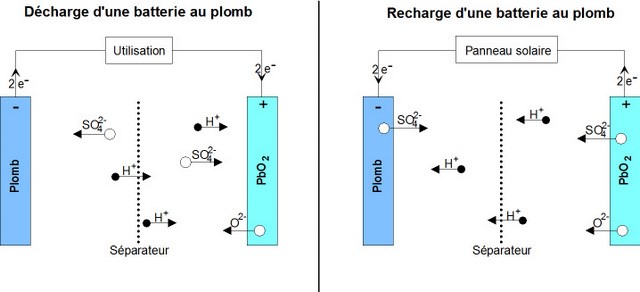
Étape 2 - Operation of a lead acid battery
To understand the causes of battery failure, it is important to understand the chemical reactions at work inside it.
Reaction during discharge: During discharge, the following chemical reaction takes place:
PbO2 sol + Pb sol + 2 HSO4−aq + 2 H+aq ⟶ 2 PbSO4 sol + 2 H2O liq
- The positive (+) electrode which is lead dioxide converts into lead sulphate crystals. (TVP: Maybe rewrite is as: The positive electrode, which is lead dioxide, reacts with the electrolyte sulphuric acid to form lead sulphate crystals and water in a reduction reaction),
- The negative electrode (-) which is made of lead also changes into lead sulphate crystals. (Or: The negative elctrode which is lead, also reacts with sulphuric acid to form lead sulphate crystals and water in an oxidation reaction).
- The electrolyte bath in which the reactions take place is largely transformed into water ((H2O).
Reaction during Charge
When charging, the reverse chemical reaction takes place: 2PbSO4 sol + 2 H2O liq ⟶ Pb sol + PbO2 sol + 2 HSO4−aq + 2 H+aq.
2PbSO4 sol + 2 H2O liq ⟶ Pb sol + PbO2 sol + 2 HSO4−aq + 2 H+aq.
- Lead sulphate crystals dissolve/(are broken down into) lead dioxide which is deposited on the (+) electrode and lead which is deposited on the (-) electrode.
- The electrolyte reverts to dilute sulfuric acid.
Étape 3 - The units of the batteries are indicated as abbreviations which are not always easy to understand. Here is a summary table of the units associated with the batteries:
Characteristic?? Unit Definition Explanation Capacity (Ah) The amount of current that a battery can store or release, usually specified in Ah for a given discharge rate. A 10 Ah battery can produce 5 Amperes (A) for 2 hours (h). Tension (V) Battery voltage level. It must be compatible with the connected devices. Lead-acid batteries are made up of units delivering 2.1 Volts (V) and connecting these units in series makes it possible to reach the generally desired voltage. For example, six units connected in series deliver 12 V. To create 24 V or 48 V systems, 12 V batteries are, in turn, connected in series. Energy (Wh) The product of multiplication of the capacity by the voltage. A 200Ah 24V battery will have an energy of 4800 Watts hour (Wh).
Discharge rate, Cxx Expressed as a unit of C10, C20 or C100, it indicates the capacity of a battery according to its rate of discharge. Here in Cx, x is the time in hours that it takes to discharge the battery. 50Ah C20 battery means a battery of 50Ah capacity with 20h discharge C100 battery: 90Ah (capacity of 90Ah with a discharge in 100h)
Cold Cracks Amps (CCA) This is the maximum extractable current from a battery over a short period when starting the engine, for example. CCA 420A 5 sec indication means the battery can deliver 420A for 5 sec
SOC (State of Charge) State of charge of a battery, which indicates the amount of electricity remaining. SOC = 50 %: the battery’s charge is 50%. DOD (Depth of Discharge) State of discharge of a battery, or the amount of electricity consumed. DOD + SOC = 100% Number of cycles For a battery, a cycle represents a discharge followed by a charge. The number of cycles of a battery depends on the depth of discharge or amount of electricity consumed. The higher the DOD, the lower the cycle life. The same battery can have:
500 cycles at 80% DOD • 750 cycles at 50% DOD • 1800 cycles at 30% DOD
{{Tuto Step |Step_Title=Different Types of Batteries for Different Uses |Step_Content=There are several types of and technologies for lead batteries, each adapted to a particular use, environment and constraints. Understanding the differences is essential to choosing and maintaining your battery correctly. This part summarizes the main categories of lead acid batteries and their characteristics.
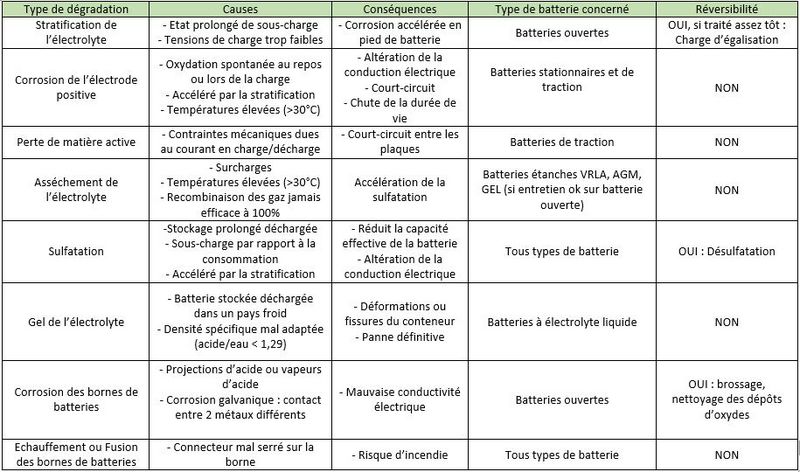
Étape 4 - Mechanisms of degradation of Lead Acid Batteries
• Stratification of the electrolyte: In a wet electrolyte battery, if the electrolyte is not agitated, the sulfuric acid will flow down the trays./plates. Thus, the density of the electrolyte will slowly increase at the bottom of the batteries, while it will decrease at the top of the batteries. This stratification of the acid will result in non-homogeneous electrode discharge with accelerated corrosion at the bottom of the battery.
How to avoid it • Use batteries regularly -- The electrolysis of water creates bubbles of oxygen which agitate the electrolyte. • Periodically carry out an equalization charge -- This consists of charging the batteries with a low current, but with higher voltage than that generally applied to create greater bubbling. • Use gel or AGM batteries.
• Corrosion of the positive electrodes: The positive electrodes are sensitive to corrosion which occurs when not in use, but especially during charging, when the lead in the grid is transformed into lead oxide, which is not very conductive. If there is too much corrosion, the active materials gradually sink to the bottom of the accumulators, and electrodes disintegrate. The capacity of the battery decreases and the internal resistance increases until the battery becomes unusable.
How to limit it? • Avoid overloads: check the data sheets so that the load currents and durations are not too high. • Avoid high temperatures: ventilate or insulate the battery room, leave a space between each battery.
• Loss of active material: During charge and discharge cycles, the positive and negative plates undergo strong mechanical stresses (high currents, induced magnetic fields). The plates gradually disintegrate and the active material accumulates at the bottom of the battery. This "mud" can cause short circuits between two plates.
How to limit it? • Avoid deep discharges. • Avoid rapid discharges: If the battery is discharged very quickly, the mechanical stresses do not have time to accommodate themselves and disintegration is faster. • Choose batteries with thick or tubular electrodes.
• Drying of the electrolyte: Naturally, the water contained in the electrolyte evaporates a little. Valve regulated lead acid (VRLA) batteries promote its recondensation, which reduces the need for additional distilled water (unlike open batteries). But, once the battery is charged, a supply of current initiates the electrolysis of the water with the formation of oxygen and hydrogen gas. In a VRLA battery, beyond a certain pressure, safety valves let the water escape permanently. This is problematic because topping up with distilled water is not possible.
How to avoid it? • Avoid overloads: check the data sheets to ensure that the load currents and durations are not too high. • Avoid high temperatures: ventilate or insulate the battery room, leave space between each battery.
• Sulfation: During discharge, crystals of lead sulfate (PbSO4) form on the positive and negative electrodes. If the battery remains discharged for a long time, these lead sulphate crystals grow and harden irreversibly. This reduces the conductivity of the electrodes, causes the battery to lose capacity and can cause short circuits.
How to limit it? • Avoid prolonged undercharging: never store a discharged battery. • Avoid incomplete charges: charge your batteries to 100% at least once a week.
• Freezing of Electrolyte: When a battery is discharged, the electrolyte is mostly water. At low temperatures, it can freeze and irreparably damage the battery.
How to avoid it? • Avoid too short daily journeys by car in winter. • In cold climates, increase the specific gravity of the electrolyte if the battery is open (acid/water = 1.29-1.3 g/cm3). • Switch to AGM or Gel batteries.
Corrosion of the battery terminals: Following acid splashes, acid vapours, or simply galvanic corrosion (two metals brought into contact), lead oxide deposits may form on the battery terminals. This can cause electrical conductivity problems.
How to avoid it? • Lubricate the connectors with petroleum jelly or an anti-corrosion grease suitable for batteries • Brush and clean the terminals if traces of corrosion appear.
• Fusion of the battery terminals: If the connector is loose on the terminal, the electrical contact resistance will increase. When a high current passes, the terminals can melt by the Joule effect (the conversion of electric energy into heat energy by resistance in a circuit). This can lead to fires.
How to avoid it? • Comply with the tightening torques in N.m given by the battery manufacturers. • Regularly check the correct tightening, especially if the batteries are subject to vibrations (for example, golf cars, trailers, etc.).
Étape 5 - Summary of good practices to adopt with lead acid batteries
• Equipment:?? Choose your battery carefully according to the intended use. Never mix new and used batteries. Never mix batteries of different technologies. Correctly and solidly install the wiring of your battery bank to avoid fires. Regularly check the connectors if they are subject to vibrations.
Detection and Prevention of Deep Discharge: Battery life is directly related to DoD or depth of discharge. It is, therefore, very important to prevent any discharge over 50%. o How to know the level of charge (SoC)? Simply measuring the voltage does not suffice as several factors affect the battery voltage. A battery monitor must be used. It calculates not only the voltage but also the charge and discharge currents, which allows the state of charge to be calculated in real time.
o How to avoid deep discharges? The idea is to control the level of charge (SoC) and to disconnect the consumption loads as soon as they fall below a certain level. Use a battery protector or a configurable solar charge regulator, for direct current (DC) equipment. Use the dry contact relay (voltage-free relay) of your battery monitor if it is equipped with one. Set the low battery voltage threshold on your inverter for alternating current (AC) equipment (read the instructions carefully).
Pay attention to the temperature: This factor has a very important influence on the life of the batteries. It is very important to keep the batteries at “cool” temperatures, around 20°C. o Technical room -- Always choose the coolest room or location. Never leave batteries exposed to direct sunlight. If the place is still too hot, one should consider cooling ventilation of the room or the battery container. o Aeration and ventilation -- Always keep space between the batteries (about 5 cm), do not put them against each other. If the batteries are inside a battery box or in a cabinet, there must be air circulation. o Temperature compensation -- When the temperature exceeds 30°C or is lower than 10°C for a long time, it is necessary to change the charging voltage.
Battery not in use – Self-discharge: When a battery is not in use, it slowly discharges. This phenomenon depends on the type of battery and the temperature. o An unused open battery must be recharged every four months at room temperature (between 10-25°C). o An unused open battery must be kept permanently charged in temperatures below 0°C. o Sealed batteries can be left for up to 6 to 8 months without recharging at ambient temperature. o When a system containing batteries (RV, car, etc.) is not used for a long period, disconnect the batteries to avoid leakage currents.
Correct charging voltages: Never recharge the batteries with a voltage higher than that recommended in the manufacturer's data sheet. Use a charger with at least 3 charge stages (Bulk, Absorption, Float).
Correct charge/discharge current: It is recommended never to charge or recharge lead batteries at more than 0.2C, i.e. 20% of the capacity of the battery bank (ex: 20A for a battery bank of 100Ah).
When sizing a photovoltaic installation, make sure that the maximum output current is less than 20% of the battery capacity. Let: Imax (A) = Pmax (W) / Ubat (V) < 0.2C
Étape 6 - Desulphation/Regeneration of lead acid batteries
During discharge, lead sulphate (PbSO4) forms on the positive and negative electrodes. If the battery remains discharged, this lead sulphate crystallizes and hardens. Once crystallized, it can no longer turn into sulfuric acid when charging the battery. This causes the battery capacity to drop: "it no longer holds a charge" it is a weak battery/it is a dead battery.
Battery regeneration is a process of sending high-intensity electrical pulses (300-400A) at a given frequency, based on the battery's resonant frequency. This is calculated automatically by the machine and evolves over time. These impulses break down the crystalline layer formed by the amorphous lead sulphate deposited on the electrodes and convert it back to sulphuric acid. The plates are reconstituted and the battery returns to its original condition.
Success rate: Since sulphation is not the only phenomenon underlying battery degradation, not all of them can be regenerated by desulphation.
• For batteries with tubular electrodes, the success rate is around 90% (source: BeEnergy) • For starter batteries, the success rate is around 30%. (source: BeEnergy)
Duration of the process: This process can last from a few hours for a starter battery to several days for traction batteries.
Research to follow (For Further Reading)
Notes et références
Document by Guénolé Conrad with the help of Loup Girier, Wiam Razi, Elliot Harant and Pascal Criquioche within the framework of the Scholar Grid project. A project initiated by the Schneider Electric Foundation with the technical support of Energie Sans Frontières, Atelier 21 and the Low-tech Lab
Document Victron Energy, Translated from: “Optimizing the life of lead batteries - Lesson V02 Bis.docx” by Margriet Leeftink, by Jacques Noël”
Summary on the Installation of lead batteries: Website: Batterie-solaire.com Summary on the Maintenance of lead batteries: Website: Batterie-solaire.com Report "State of the Art of Desulphation Technologies for Lead-Acid Accumulators" by ADEME - 2011 Video "Liquid battery, AGM, GEL, what to choose?" from the Youtube channel of Guillaume Piton - La Watterie
Published

 Français
Français English
English Deutsch
Deutsch Español
Español Italiano
Italiano Português
Português