Description
This tutorial gives you the process on how to make biodiesel on a medium-scale (about 50L) from used cooking oil. This tutorial was written following the Nomade des Mers crew's visit to Cambodia. The social enterprise Naga Earth collects and converts used cooking oil from the town's hotels and restaurants into biodiesel. They also make soap with glycerin that comes from this same reaction. This benefits various schools and associations who carry out hygiene programs throughout the region.
Sommaire
Sommaire
- 1 Description
- 2 Sommaire
- 3 Introduction
- 4 Video d'introduction
- 5 Étape 1 - Identify your needs
- 6 Étape 2 - Filtration
- 7 Étape 3 - Titrage de l’huile (seulement pour les huiles usagées)
- 8 Étape 4 - Transestérification
- 9 Étape 5 - Séparation du glycérol
- 10 Étape 6 - Lavage et séchage du biodiesel
- 11 Étape 7 - Traitement du glycérol
- 12 Notes et références
- 13 Commentaires
Introduction
Biodiesel is an alternative fuel to petro-sourced diesel. It can be used alone in engines or blended with petro diesel with different concentration levels. This fuel is obtained from vegetable oil or animal fat that is converted by a chemical process named "transesterification". It involves making oil react with an alcohol (methanol or ethanol) and a catalyst (sodium or potassium hydroxide) in order to obtain methyl or ethyl esters (biodiesel) and a by-product called glycerin.
Biodiesel can be made in various amounts. The processes described here are suitable for occasional production and small amounts. Because the process requires practice, we recommend you start by making small amounts then gradually go towards a larger scale of production.
"Biodiesel has man benefits, making it an interesting fuel alternative:" *It is simple to make yourself. *It can be produced at a low cost* It can be used in any conventional diesel engine. It also allows for better lubrication of the engine. *It contributes to the recycling of organic waste, such as used cooking oil that is widely used in restaurants. *It is made from vegetable oil and therefore releases only a small additional amount of CO2 into the atmosphere. It also reduces the emissions of certain harmful compounds compared to petro-diesel (carbon monoxide, sulphur dioxide, etc)
Safety measures
- Wear safety glasses, a gown, resistant gloves and long clothes. Working with a breathing mask is also recommended.
- Methanol is the most dangerous product in the making of biodiesel. It's very flammable and the slightest spark could cause burns or an explosion. It is also toxic and can cause blindness if inhaled or ingested.
- Sodium hydroxide (soda - NaOH) and potassium hydroxide (caustic potash - KOH) are corrosive products, avoid all skin contact (if skin contact should occur, rinse with vinegar then with water).
- Work with an extinguisher nearby.
- Work in a well-ventilated area (to reduce the risk of toxic vapours).
- Work near a sink and a source of running water.If you wish to reduce both your consumption and expenses on fossil fuel, there are several options available: *vegetable oil fuel blended with diesel *L'huile végétale carburantwith engine modification *Le Biodiesel Although this tutorial describes the third option, it's important to consider the two other options beforehandThe first step is therefore dedicated to the different considerations to be taken into account before choosing. }}
Youtube
Matériaux
"1.1 Oil"
Our approach being the most ecological possible, the oil used for making biodiesel is "used cooking oil" collected from all sorts of eating establishments. In order to collect the best sort of oil, talk to the owner of the restaurant as well as the chef so you can explain the purpose of your approach. Collecting oil without permission can be qualified as an offence. Furthermore, make sure you check the quality of the oil collected. Used oil contains free fatty acids. They appear when oil is left in the open air over a certain period of time and becomes rancid or when heated in the presence of water. Frying food (that contains water) therefore causes FFAs to appear in oil. During transesterification, these FFAs react with the catalyst (strong base) and create an unwanted soap. The soap stops the products of the reaction, biodiesel and glycerol, from separating correctly, which creates an emulsion that is difficult to work with.
The more the cooking oil is used, the more the concentration in FFAs increases and becomes browner. "Therefore, try to avoid collecting old, brown oil". The better the quality of the oil is, the easier the converting process will be.
When making biodiesel, "prefer" using oil such as vegetable oil with a neutral pH or nearly neutral such as "rapeseed, corn oil or sunflower oil". These oils have a low freezing point, which means they don't solidify if temperatures drop in winter (freezing temperatures for these oils range from -10°C to -2°C for rapeseed, -15°C for sunflower).
"Avoid peanut, coconut and palm oil or animal fats" because the temperatures at which they solidify are too high (>10°C), even if biodiesel has a lower freezing point than the oil it originates from. Also ban olive oil that is too acidic, these acids could interfere during the reaction in the creating of biodiesel.
1.2 Methanol
Methanol, or wood alcohol, used to be obtained by wood pyrolysis. Nowadays, it is synthesized from natural gas and is mainly used as an antifreeze for coolants, as a solvent for the synthesis of other chemicals or as fuel in the racing field (dragsters, model engines). It can be purchased through suppliers who specialize in chemicals, some DIY stores and garages.
1.3 Catalyst
Either sodium hydroxide (NaOH, caustic soda) or potassium hydroxide (KOH, caustic potash) can be used as a catalyst. They are both corrosive and highly basic. These chemicals are quite common and can be found in DIY stores, on line or through chemical suppliers. KOH and NaOH are both hygroscopic, which means they absorb the humidity in the atmosphere quickly. They become less efficient catalysts in the presence of water: always keep them in sealed, airtight containers.
NaOH vs KOH, different properties."
- Sodium hydroxide (NaOH) is less expensive and commonly used to make biodiesel on a small scale. However, sodium hydroxide (NaOH) contaminates the water used for cleaning biodiesel and must not be disposed of in nature. We therefore suggest you don't use it if you cannot ensure water treatment.
- Potassium hydroxide (KOH) having higher catalysis properties, is gaining in popularity. Potassium hydroxide (KOH) dissolves easier in methanol and is less sensitive to water. Furthermore, the glycerin obtained through the reaction remains liquid and can be added to compost with more safety or used in small amounts as a supplement for animal feed.
Outils
Transesterification * Used vegetable oil Modèle:For information * 250 mL of methanol/ litre of oil * If you are using new oil : 5,5g of NaOH or 7g of KOH / litre of oil. *If you are using used oil: Calculate the catalyst mass that must be added when you get to the titration stage. Modèle:For information * 1 clean, dry container with a drain tube or a tap for when you want to carry out the reaction Modèle:For information
- • 1 submersible pump so you can do the blending in the canister * - 1 one hot plate or heating resistor * - 1 temperature sensor * - 1 precision balance * - 1 funnel * - 1 big glass jar shut or a stainless steel tank for the blending of the methanol and the catalyst * - 1 measuring glass for the dosage of methanol* - rubber gloves * - "Filtration" safety glasses * - 1 canister of new oil * - 1 clean and empty oil canister * - 1 funnel * - 1 cotton sheet/ 1 sock * - a 1 to 5µm "Titration" filter bag" * - 10ml of isopropyl alcohol or isopropanol. * -1ml of your used oil * - 2-3 drops of phenolphthalein (indicator) * - 1L of distilled water * - 1g of catalyst, sodium hydroxide NaOH or potassium hydroxide KOH. •
Étape 1 - Identify your needs
There are at least three ways of making a diesel engine run with vegetable oil: *blending it with petro-diesel, a solvent or petrol; *using it as it is - usually called huile végétale carburant (HVC, SVO in English) or recycled vegetable oil (HVR, WVO in English); *converting it into biodiesel. The two first methods look easy, but as for many things, it isn’t that simple.
1. Blends"
Vegetable oil is a lot more viscous (thicker) than petro-diesel or biodiesel The purpose of blending or mixing SVOs with other fuels or solvents is to reduce the viscosity to make it thinner, so it will flow easier through the fuel system to the combustion chamber. However, this is not the only problem when using SVOs. Its chemical properties and combustion characteristics are different to those of petro-diesel, for which diesel engines and their fuel systems are designed for. Diesel engines, especially the most modern ones, are high-tech machines with specific fuel needs (see la controverse TDI-SVO).
People use various blends, ranging from 10% SVO and 90% petro-diesel to 90% SVO and 10% petro-diesel. Some people use it as it is, without preheating (which makes the vegetable oil a lot more viscous). Some even use 100% SVO without preheating. They often don't realize the long term effects on the engine
You might manage it during the summer period with something like an old 5 cylinder Mercedes-Benz that's over 80 years old, that has a very resistant and tolerant engine. It won't like it but you probably won't destroy the engine. Otherwise, it probably isn't a good idea.
I can't guarantee it but using a blend containing up to 20% of good quality vegetable oil with 80% of petro-diesel is known to be quite safe for old diesels, especially in summer.
If you blend SVO with petro-diesel, you'll still be using fossil fuels - so you'll be cleaner than average, but still not enough for others. Yet, for each litre of SVO you use, you save one litre of fossil fuel and lower carbon dioxide emissions. dioxyde de carbone
However, in order to do it correctly and safely all year round, you will need a system suitable for SVO, including a preheating fuel system at the least (see below) and in that case, blending isn't needed, you can simply use 100% SVO.
Lastly, blending SVOs with other solvents like mineral turpentine (white spirit) or other "secret" ingredients such as naphthalene or xylol or with unleaded petrol are only experimental processes at this point in time and the effects of these additives on the characteristics of fuel combustion or their long term effects on the engine remain unknown. We highly recommend you don't choose this kind of solution: use such blends at your own risk.
2. Huile végétale carburant avec modification du moteur
Direct SVO fuel systems can be a clean, efficient and economical option.
Unlike biodiesel, you will need to modify your engine so you can use SVO. The best way to get one is to purchase a professional single-tank SVO system [1] with optimized injector nozzles, glow-plugs for vegetable oil as well as a fuel pre-heating system. With this système Elsbett system, you can use petro-diesel, biodiesel or SVO, with any combination you like. Start up and go, turn off and stop, just like any other car.
There are other SVO systems with two tanks that preheat the oil to make it less viscous. You must start your engine up with ordinary petro-diesel ( or biodiesel) in the tank, then switch to SVO in the other tank once the vegetable oil is hot enough (so quite liquid) then come back to petro-diesel before turning the engine off, or you'll clog the injectors.
You can find lots of information about SVO systems on here.
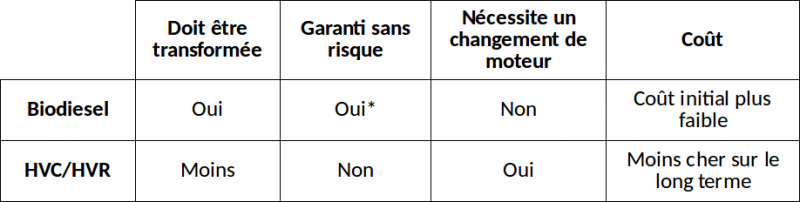
3 Biodiesel or SVO?"
Le biodiesel présente des avantages évidents sur SVO:
- Il fonctionne dans n'importe quel diesel, sans aucune conversion ou modification du moteur ou du système d'alimentation - il suffit de le mettre dans le réservoir et démarrer.
- Il a également de meilleures propriétés en temps froid que l'HVC (mais pas aussi bonnes que le pétro-diesel - voir ici pour l'utilisation du biodiesel en hiver).
- Contrairement à l'HVC, il est soutenu par de nombreux tests sur le long terme dans de nombreux pays, avec des millions de kilomètres de route parcourus.
Le biodiesel est un carburant de remplacement propre, sûr, prêt à l'emploi, tandis que de nombreux systèmes HVC sont encore expérimentaux et doivent être développés.
D'un autre côté, le biodiesel peut être plus coûteux, en fonction de la quantité que vous produisez, de ce que vous obtenez et de la comparaison avec de l'huile ou du pétrole neuf. Et contrairement à l'HVC, il nécessite un traitement (l'objet de ce tutoriel). Mais la communauté, qui s'étend de plus en plus rapidement, de producteurs de biodiesel ne se préoccupe pas de cela - ils font une fournée chaque semaine ou chaque mois et ils s'y habituent rapidement. Beaucoup le font depuis des années.
Quoi qu'il en soit, vous devez également traiter l'HVC, en particulier l'HVR, que beaucoup de personnes utilisent avec des systèmes HVC parce qu'elles sont bon marché voire gratuites. Avec l'HVR, les particules alimentaires et les impuretés et l'eau doivent être enlevées, et elle devrait également être désacidifiée. Les producteurs de biodiesel pensent : «Si je dois faire tout ça, autant faire du biodiesel à la place». Mais les défenseurs de l'HVC soutiendront que c'est beaucoup moins de traitement que le biodiesel.
Les goûts et les couleurs... à vous de juger !
Ci-contre un tableau résumant les principales différences entre faire du biodiesel et modifier son moteur pour utiliser directement de l'HVC. SVO/WVO sont les correspondances anglophones de HVC/HVR.
$ Considérations financières : $
Un français consomme en moyenne : 1,3[€/L]*4/100[L/km]*19000[km/an] = 988 €/an Soit environ 1000 € par an de diesel.
Le biodiesel coûte entre 0,5 et 1 € par litre à produire à partir d'huile recyclée, soit entre 380 et 760 € par an. Un bon système de traitement adapté à ce volume peut être construit pour environ 100€. Celui-ci est donc remboursé en quelques mois.
Un système pour HVC coûte entre 900 et 1800 €. Si l'HVC est gratuite, celui-ci sera donc remboursé en 1 à 2 ans.
Étape 2 - Filtration
Avant de pouvoir être transformée, l’huile usagée doit tout d’abord être filtrée pour la nettoyer des éventuels résidus alimentaires (frites, beignets…). Il est conseillé de laisser l’huile décanter pendant quelques jours. Filtrer une première fois l’huile pour se débarrasser des particules les plus grosses (ex: drap en coton doublé, chaussette…). Puis verser l’huile dans une poche de filtration d'au maximum 5µm (le plus fin sera le mieux) au-dessus d’un bidon propre prévu à cet effet.
Étape 3 - Titrage de l’huile (seulement pour les huiles usagées)
- Peser précisément 1g de catalyseur et le dissoudre dans 1L d’eau distillée
- Dans un récipient gradué en verre, mesurer 10 mL d’isopropanol.
- Prélever 1 mL de votre huile à tester à l’aide d’une seringue graduée et ajouter à l’isopropanol.
- Ajouter 3 gouttes de phénolphtaléine au mélange.
- Bien remuer le mélange, si possible avec un agitateur magnétique.
- Remplir la burette graduée, préalablement rincée à l’eau distillée, de 10 mL du mélange eau distillée/catalyseur.
- Ajouter la solution eau distillée-catalyseur au mélange, goutte par goutte, tout en remuant continuellement.
- Continuer l’ajout de solution titrante peu à peu jusqu’au point de virage. Le mélange devient de couleur rose foncé. Le changement de couleur doit persister pendant 20 secondes.
- Relever exactement le volume d’eau distillée/catalyseur (Vtitrage) utilisée pour neutraliser l’acidité de l’huile.
- Refaire un deuxième essai et calculer la moyenne des essais pour plus de précision.
- Utiliser cette information pour connaître la quantité de catalyseur à ajouter dans la réaction suivant la formule : (Pour comprendre son obtention voir l'ANNEXE 2 de ce document)
Masse catalyseur requise (g) = (B+ Vtitrage(mL)) x Volume huile (L)
Avec :
Vtitrage : volume de solution titrante ajoutée pour neutraliser l'acidité de l'huile (mL)
B : la quantité de base (catalyseur) nécessaire pour la réaction avec de l’huile vierge.
B (KOH) = 7.0 g/L
B (NaOH) = 5.5 g/L
Étape 4 - Transestérification
- Faire chauffer l’huile à 60°C et la maintenir à cette température.
- Verser le méthanol dans un récipient en verre.
- Peser la quantité de soude définie au cours de l’étape précédente si vous utilisez de l’huile usagée et ajouter au méthanol.
- Remuer et laisser se dissoudre entièrement le catalyseur dans le méthanol. Cela prend environ 2min. Encore une fois, attention : le mélange se dégrade rapidement. Procéder à l’étape suivante dès que le mélange catalyseur/méthanol est homogène.
- Ajouter le mélange catalyseur/méthanol à l’huile chaude (60°C). Laisser les éléments se mélanger pendant plusieurs heures. Lors de la réaction, deux produis sont formés : le biodiesel et le glycérol. Le biodiesel étant moins dense, il forme la couche supérieure.
- Attendre 24 heures ou plus avant de procéder à l’étape suivante.
Étape 5 - Séparation du glycérol
Drainer le glycérol par le bas de la cuve dans un nouveau contenant. Faite attention à ce qui ne reste plus de glycérine dans le biodiesel. Conserver le glycérol obtenu.
Étape 6 - Lavage et séchage du biodiesel
- Ajouter très lentement 20 % du volume de biodiesel en solution de lavage pour éviter la formation de savon et attendre 10 min. Drainer l’eau de lavage.
- Procéder à un second lavage. Cette fois, retourner le contenant lentement 4 à 5 fois et attendre 10 minutes. Une agitation excessive favorise la formation d’émulsion et provoquera un délai d’attente supplémentaire. Drainer l’eau de lavage.
- Procéder à un troisième lavage. Cette fois, agiter énergiquement le contenant pendant quelques minutes. Laissez reposer puis drainer l’eau de lavage.
- Transférer le biodiesel vierge et lavé dans un nouveau contenant.
- Chauffer à 50 °C pendant quelques heures jusqu’à ce que le biodiesel devienne clair.
Étape 7 - Traitement du glycérol
La récupération du méthanol contenu peut s’effectuer par distillation du glycérol, c’est-à-dire en chauffant le glycérol à une température supérieure à 65°C, le point d’ébullition du méthanol. Les vapeurs de méthanol doivent ensuite passer au travers d’un condenseur, refroidir et être récoltées sous forme de liquide en sortie.Tuto vidéo de construction d'un alambic maison: https://www.youtube.com/watch?v=5H1UVv8FaO8
- Former un serpentin avec le tuyau de cuivre (Par exemple autour d'un bidon d'eau). Laisser les deux extrémités rectilignes sur environ 30 cm.
- Fixer le tuyau flexible sur la sortie de la cocotte minute à l'aide d'un collier de serrage.
- Réaliser la jonction entre le tube de cuivre et le tuyau flexible à l'aide de colliers de serrage.
- Verser la glycérine dans la cocotte minute et chauffer l'ensemble à au moins 100 degrès. Le glycérol ayant une température d’ébullition de 290°C, pas besoin de contrôler précisément la température.
- Refroidir le serpentin de cuivre en le plongeant dans un bac d'eau ou avec un linge humide pour accélérer la condensation du méthanol.
- Récolter le méthanol recondensé dans un bocal en verre.
Notes et références
https://attra.ncat.org/attra-pub-summaries/?pub=318
http://journeytoforever.org/biodiesel_make.html#start
http://carburerauxalgues.com/site/download/download_file_st/ActiviteI_ELEVES/pdf
http://www.make-biodiesel.org/
Published






















 Français
Français English
English Deutsch
Deutsch Español
Español Italiano
Italiano Português
Português