(Page créée avec « *Solder *A heating system (min. 400°C) *butane/propane gas canister (375mL) ») |
(Mise à jour pour être en accord avec la nouvelle version de la source de la page) |
||
| (11 révisions intermédiaires par 2 utilisateurs non affichées) | |||
| Ligne 1 : | Ligne 1 : | ||
| − | {{ | + | {{Tuto Details |
|Main_Picture=Pyrolyseur_de_plastique_IMG_8544.JPG | |Main_Picture=Pyrolyseur_de_plastique_IMG_8544.JPG | ||
|Description=Produce fuel from plastic | |Description=Produce fuel from plastic | ||
| − | |Area=Energy | + | |Area=Energy |
|Type=Prototype | |Type=Prototype | ||
|Difficulty=Medium | |Difficulty=Medium | ||
| Ligne 14 : | Ligne 14 : | ||
|IsTranslation=1 | |IsTranslation=1 | ||
}} | }} | ||
| − | {{ | + | {{Introduction |
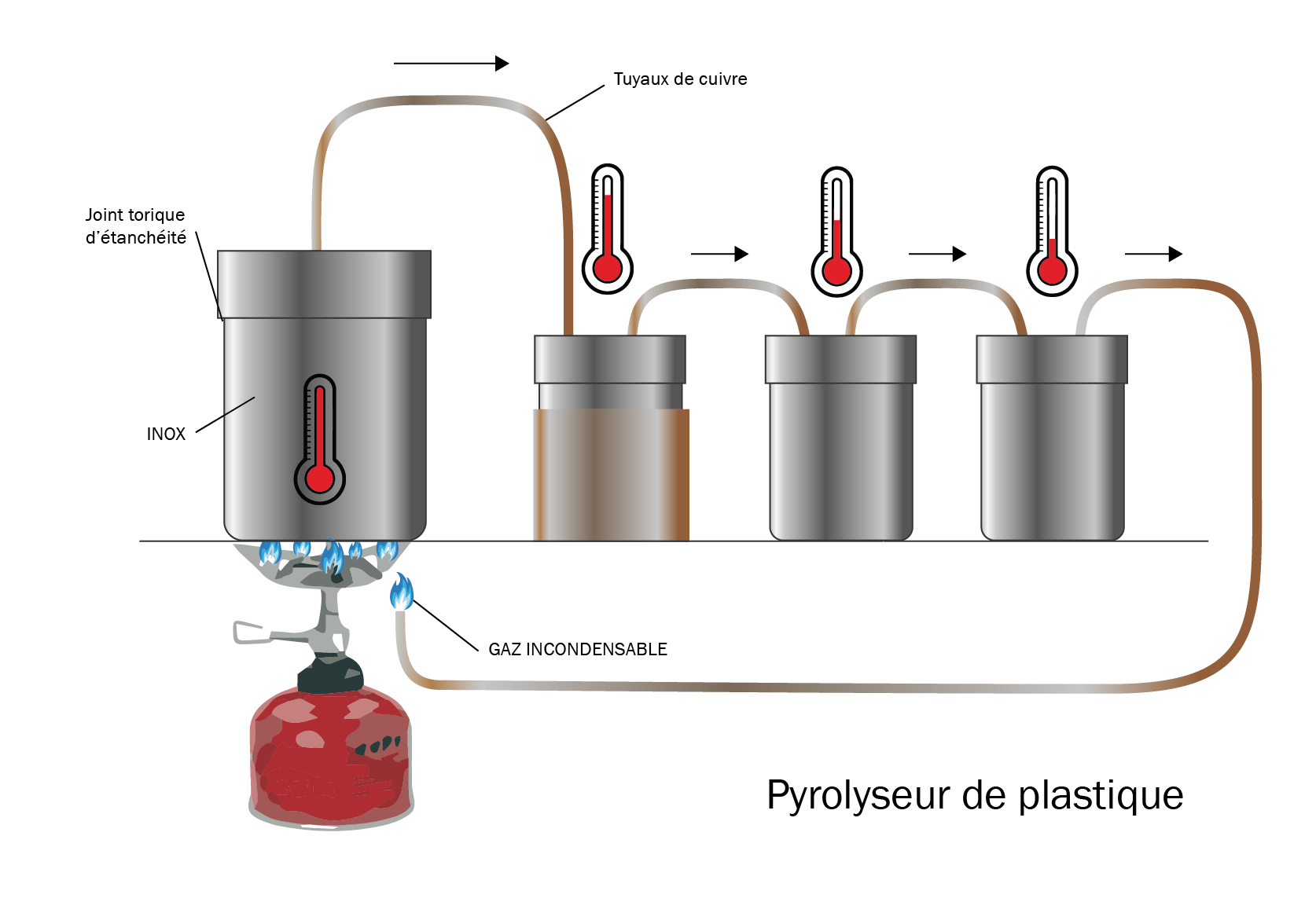
|Introduction=Plastic pyrolysis is a distillation process that allows plastic waste to be converted into fuel. Plastic waste is heated above 400°C in a first tank into a gas. Depending on condensation (cooling) temperatures, several types of fuel are produced : | |Introduction=Plastic pyrolysis is a distillation process that allows plastic waste to be converted into fuel. Plastic waste is heated above 400°C in a first tank into a gas. Depending on condensation (cooling) temperatures, several types of fuel are produced : | ||
- between 390 and 170°C, the gas condensates into diesel fuel. | - between 390 and 170°C, the gas condensates into diesel fuel. | ||
| Ligne 22 : | Ligne 22 : | ||
In this prototype, we are only using polypropylene (PP) and/or high density polyethylene (HDPE) and low density (LDPE). Please note that using mostly polypropylene will produce more gasoline, while using mostly polyethylene will produce more diesel fuel. It is however possible to mix both. | In this prototype, we are only using polypropylene (PP) and/or high density polyethylene (HDPE) and low density (LDPE). Please note that using mostly polypropylene will produce more gasoline, while using mostly polyethylene will produce more diesel fuel. It is however possible to mix both. | ||
}} | }} | ||
| − | {{ | + | {{Materials |
|Step_Picture_00=Pyrolyseur_de_plastique_Capture_d_e_cran_2017-12-05_a_19.07.21.png | |Step_Picture_00=Pyrolyseur_de_plastique_Capture_d_e_cran_2017-12-05_a_19.07.21.png | ||
|Step_Picture_01=Pyrolyseur_de_plastique_106597-bisphenol-a-decryptez-les-etiquettes-pour-eviter-les-produits-dangereux-622x0-1.jpg | |Step_Picture_01=Pyrolyseur_de_plastique_106597-bisphenol-a-decryptez-les-etiquettes-pour-eviter-les-produits-dangereux-622x0-1.jpg | ||
| Ligne 35 : | Ligne 35 : | ||
*butane/propane gas canister (375mL) | *butane/propane gas canister (375mL) | ||
}} | }} | ||
| − | {{ | + | {{Tuto Step |
| − | |Step_Title= | + | |Step_Title=Pack the first tank with plastic |
| − | |Step_Content= | + | |Step_Content=For this test, plastic waste is mostly polypropylene. |
|Step_Picture_00=Pyrolyseur_de_plastique_IMG_0925.JPG | |Step_Picture_00=Pyrolyseur_de_plastique_IMG_0925.JPG | ||
}} | }} | ||
| − | {{ | + | {{Tuto Step |
| − | |Step_Title= | + | |Step_Title=Preheat the second tank |
| − | |Step_Content= | + | |Step_Content=Preheating is necessary. It allows for the condensation of gases at high temperature before passing through the last two tanks. |
|Step_Picture_00=Pyrolyseur_de_plastique_IMG_0928.JPG | |Step_Picture_00=Pyrolyseur_de_plastique_IMG_0928.JPG | ||
}} | }} | ||
| − | {{ | + | {{Tuto Step |
| − | |Step_Title= | + | |Step_Title=Formation of residual gas |
| − | |Step_Content= | + | |Step_Content=Let plastic waste consume itself until non-condensable gas forms. It comes as an addition to the gas initially used. For this test, 125mL of canister gas has been used, to which has been added residual gas. |
|Step_Picture_00=Pyrolyseur_de_plastique_IMG_0931.JPG | |Step_Picture_00=Pyrolyseur_de_plastique_IMG_0931.JPG | ||
}} | }} | ||
| − | {{ | + | {{Tuto Step |
| − | |Step_Title= | + | |Step_Title=Retrieval of fuel |
| − | |Step_Content= | + | |Step_Content=Here, the machine was heated during roughly one hour. Switch off the system and let it cool down before opening the tanks. We get around 125mL of fuel in tank n°2 and 30mL of fuel in tank n°3. |
| − | * | + | *Result of test to confirm in the lab |
|Step_Picture_00=Pyrolyseur_de_plastique_IMG_0954.JPG | |Step_Picture_00=Pyrolyseur_de_plastique_IMG_0954.JPG | ||
}} | }} | ||
| − | {{ | + | {{Notes |
| − | |Notes=https:// | + | |Notes=https://en.wikipedia.org/wiki/Pyrolysis |
}} | }} | ||
| − | {{ {{ | + | {{PageLang |
| + | }} | ||
| + | {{Tuto Status | ||
|Complete=Published | |Complete=Published | ||
}} | }} | ||
| − | {{ | + | {{Separator}} |
Version du 16 décembre 2019 à 21:36
Description
Produce fuel from plastic
Introduction
Plastic pyrolysis is a distillation process that allows plastic waste to be converted into fuel. Plastic waste is heated above 400°C in a first tank into a gas. Depending on condensation (cooling) temperatures, several types of fuel are produced : - between 390 and 170°C, the gas condensates into diesel fuel. - between 210 and 20°C, the gas condensates into gasoline. - below 20°C, there remains non-condensable residual gas that can be burned to provide heat to the process.
In this prototype, we are only using polypropylene (PP) and/or high density polyethylene (HDPE) and low density (LDPE). Please note that using mostly polypropylene will produce more gasoline, while using mostly polyethylene will produce more diesel fuel. It is however possible to mix both.
Matériaux
- 1 large stainless steel tank with lid
- 3 small stainless steel tanks
- copper pipes (diameter 6mm)
- 7 copper uniseal joint (for watertightness of joints)
- O-ring (for watertightness of tanks)
- Plastic waste PP and/or HDPE/LDPE
Outils
- Solder
- A heating system (min. 400°C)
- butane/propane gas canister (375mL)
Étape 2 - Preheat the second tank
Preheating is necessary. It allows for the condensation of gases at high temperature before passing through the last two tanks.
Étape 3 - Formation of residual gas
Let plastic waste consume itself until non-condensable gas forms. It comes as an addition to the gas initially used. For this test, 125mL of canister gas has been used, to which has been added residual gas.
Étape 4 - Retrieval of fuel
Here, the machine was heated during roughly one hour. Switch off the system and let it cool down before opening the tanks. We get around 125mL of fuel in tank n°2 and 30mL of fuel in tank n°3.
- Result of test to confirm in the lab
Notes et références
https://en.wikipedia.org/wiki/Pyrolysis
Published





 Français
Français English
English Deutsch
Deutsch Español
Español Italiano
Italiano Português
Português