Description
Batteries are central/the key and expensive elements in stand-alone installations. However, their operation and maintenance are not well known/not well understood by the general public. This tutorial, therefore, has several objectives:
- Present/Explain how a lead-acid battery works.
- Present/Explain the different types of lead acid batteries
- Present/Explain the major causes of degradation of lead batteries.
- Present/Explain the rules for the use and maintenance of lead batteries.
- Introduce the process of desulfation (or regeneration) of lead batteries.
- Present/Explain how a lead-acid battery works.
- Present/Explain the different types of lead acid batteries
- Present/Explain the major causes of degradation of lead batteries.
- Present/Explain the rules for the use and maintenance of lead batteries.
- Introduce the process of desulfation (or regeneration) of lead batteries.
Sommaire
Sommaire
- 1 Description
- 2 Sommaire
- 3 Introduction
- 4 Étape 1 - Constitution d'une batterie au plomb
- 5 Étape 2 - Operation of a lead acid battery
- 6 Étape 3 - The units of the batteries are indicated as abbreviations which are not always easy to understand. Here is a summary table of the units associated with the batteries:
- 7 Étape 4 - Mechanisms of degradation of Lead Acid Batteries
- 8 Étape 5 - Summary of good practices to adopt with lead acid batteries
- 9 Étape 6 - Désulfatation / Régénération de batteries au plomb
- 10 Notes et références
- 11 Commentaires
Introduction
Batteries are often the most expensive and most fragile constituents of an electrical conversion system. Hence, it is important to take care of them through proper use and monitoring.
Lead acid batteries are very fragile. They are sensitive to overcharging, partial charging, deep discharges, excessively rapid charges, and to temperatures above 20°C. All these factors can lead to premature aging, mainly due to a combination of lack of technical knowledge, poorly- sized systems and erroneous use by a person. If one does not control these factors, the batteries will quickly be damaged.
The damage will result in reduced battery life and, in some cases, there could be irreparable deterioration of batteries. Batteries will last longer when used properly, and so their replacement will be less frequent. In the long run, one can make considerable savings. Another interesting aspect is that the conversion system will be more efficient if the batteries are in a good condition. The better the batteries’ condition, the more efficient the installation will be.
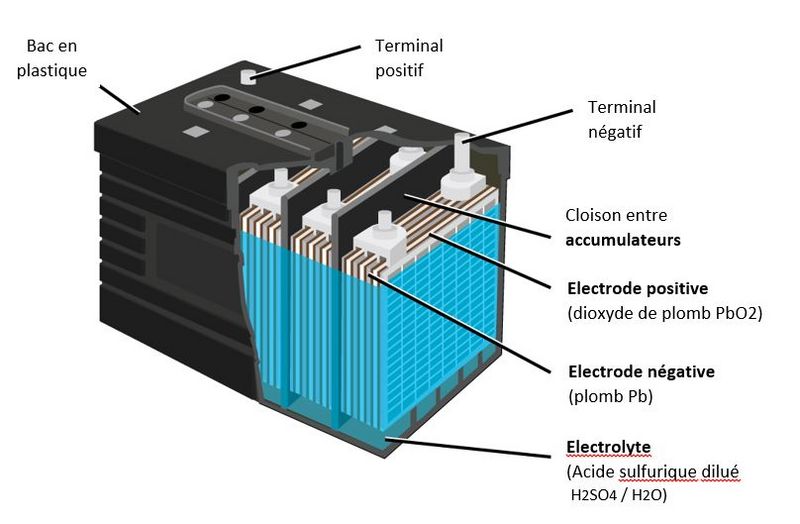
Étape 1 - Constitution d'une batterie au plomb
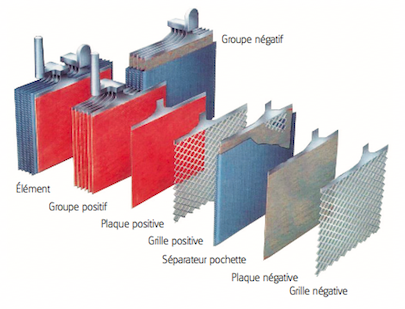
• A lead battery is made up of a set of accumulators./cells. The nominal voltage of an accumulator/cell is approximately 2.1 V, and so a 12-V battery consists of six accumulator/cell mounted in series and connected by welded lead. (A series of cells connected in series, or parallel is called module) The accumulators/cells are fitted/packed in a plastic container and sealed with a lid. • Each accumulator comprises pairs of positive and negative electrodes (plates) connected in parallel, with a separator in between each pair. • The separators are generally rectangular sheets, inserted between the positive plates and the negative plates, and have the following important characteristics: o they serve/act as perfect electrical insulators. o they are highly permeable to ions carrying electrical charges. o they have excellent resistance to sulfuric acid,
• The electrodes are composed of a grid on which is deposited a porous active material: lead (Pb) on the negative electrode and lead dioxide (PbO2) on the positive electrode. The grid collects the current and also serves as a mechanical support for the active material. • The electrolyte is a dilute solution of sulfuric acid in which the electrodes are immersed. It can be in liquid, gel or absorbed form in fiberglass felts, depending on the type of battery.
Étape 2 - Operation of a lead acid battery
To understand the causes of battery failure, it is important to understand the chemical reactions at work inside it.
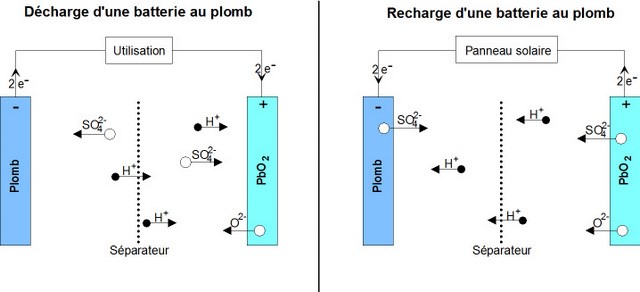
Reaction during discharge: During discharge, the following chemical reaction takes place:
PbO2 sol + Pb sol + 2 HSO4−aq + 2 H+aq ⟶ 2 PbSO4 sol + 2 H2O liq
o The positive (+) electrode which is lead dioxide converts into lead sulphate crystals. (TVP: Maybe rewrite is as: The positive electrode, which is lead dioxide, reacts with the electrolyte sulphuric acid to form lead sulphate crystals and water in a reduction reaction), o The negative electrode (-) which is made of lead also changes into lead sulphate crystals. (Or: The negative elctrode which is lead, also reacts with sulphuric acid to form lead sulphate crystals and water in an oxidation reaction). o The electrolyte bath in which the reactions take place is largely transformed into water ((H2O).
Reaction during Charge
When charging, the reverse chemical reaction takes place: 2PbSO4 sol + 2 H2O liq ⟶ Pb sol + PbO2 sol + 2 HSO4−aq + 2 H+aq.
2PbSO4 sol + 2 H2O liq ⟶ Pb sol + PbO2 sol + 2 HSO4−aq + 2 H+aq.
Lead sulphate crystals dissolve/(are broken down into) lead dioxide which is deposited on the (+) electrode and lead which is deposited on the (-) electrode.
o The electrolyte reverts to dilute sulfuric acid.
Étape 3 - The units of the batteries are indicated as abbreviations which are not always easy to understand. Here is a summary table of the units associated with the batteries:
Characteristic?? Unit Definition Explanation Capacity (Ah) The amount of current that a battery can store or release, usually specified in Ah for a given discharge rate. A 10 Ah battery can produce 5 Amperes (A) for 2 hours (h). Tension (V) Battery voltage level. It must be compatible with the connected devices. Lead-acid batteries are made up of units delivering 2.1 Volts (V) and connecting these units in series makes it possible to reach the generally desired voltage. For example, six units connected in series deliver 12 V. To create 24 V or 48 V systems, 12 V batteries are, in turn, connected in series. Energy (Wh) The product of multiplication of the capacity by the voltage. A 200Ah 24V battery will have an energy of 4800 Watts hour (Wh).
Discharge rate, Cxx Expressed as a unit of C10, C20 or C100, it indicates the capacity of a battery according to its rate of discharge. Here in Cx, x is the time in hours that it takes to discharge the battery. 50Ah C20 battery means a battery of 50Ah capacity with 20h discharge C100 battery: 90Ah (capacity of 90Ah with a discharge in 100h)
Cold Cracks Amps (CCA) This is the maximum extractable current from a battery over a short period when starting the engine, for example. CCA 420A 5 sec indication means the battery can deliver 420A for 5 sec
SOC (State of Charge) State of charge of a battery, which indicates the amount of electricity remaining. SOC = 50 %: the battery’s charge is 50%. DOD (Depth of Discharge) State of discharge of a battery, or the amount of electricity consumed. DOD + SOC = 100% Number of cycles For a battery, a cycle represents a discharge followed by a charge. The number of cycles of a battery depends on the depth of discharge or amount of electricity consumed. The higher the DOD, the lower the cycle life. The same battery can have:
500 cycles at 80% DOD • 750 cycles at 50% DOD • 1800 cycles at 30% DOD
{{Tuto Step |Step_Title=Different Types of Batteries for Different Uses |Step_Content=There are several types of and technologies for lead batteries, each adapted to a particular use, environment and constraints. Understanding the differences is essential to choosing and maintaining your battery correctly. This part summarizes the main categories of lead acid batteries and their characteristics.
Étape 4 - Mechanisms of degradation of Lead Acid Batteries
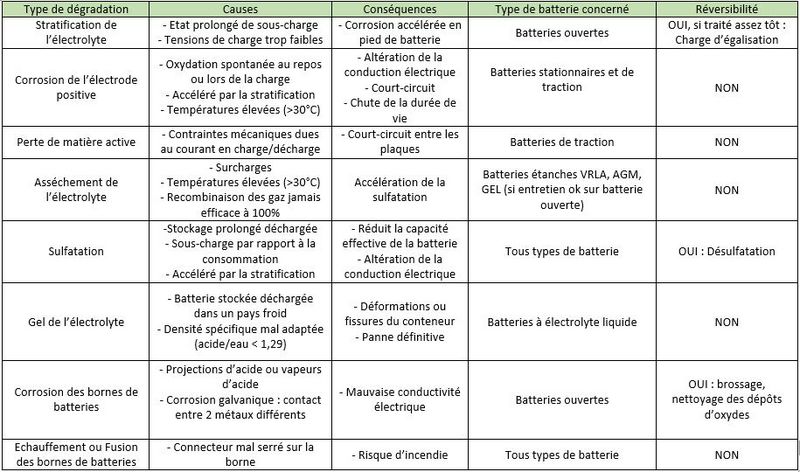
• Stratification of the electrolyte: In a wet electrolyte battery, if the electrolyte is not agitated, the sulfuric acid will flow down the trays./plates. Thus, the density of the electrolyte will slowly increase at the bottom of the batteries, while it will decrease at the top of the batteries. This stratification of the acid will result in non-homogeneous electrode discharge with accelerated corrosion at the bottom of the battery.
How to avoid it • Use batteries regularly -- The electrolysis of water creates bubbles of oxygen which agitate the electrolyte. • Periodically carry out an equalization charge -- This consists of charging the batteries with a low current, but with higher voltage than that generally applied to create greater bubbling. • Use gel or AGM batteries.
• Corrosion of the positive electrodes: The positive electrodes are sensitive to corrosion which occurs when not in use, but especially during charging, when the lead in the grid is transformed into lead oxide, which is not very conductive. If there is too much corrosion, the active materials gradually sink to the bottom of the accumulators, and electrodes disintegrate. The capacity of the battery decreases and the internal resistance increases until the battery becomes unusable.
How to limit it? • Avoid overloads: check the data sheets so that the load currents and durations are not too high. • Avoid high temperatures: ventilate or insulate the battery room, leave a space between each battery.
• Loss of active material: During charge and discharge cycles, the positive and negative plates undergo strong mechanical stresses (high currents, induced magnetic fields). The plates gradually disintegrate and the active material accumulates at the bottom of the battery. This "mud" can cause short circuits between two plates.
How to limit it? • Avoid deep discharges. • Avoid rapid discharges: If the battery is discharged very quickly, the mechanical stresses do not have time to accommodate themselves and disintegration is faster. • Choose batteries with thick or tubular electrodes.
• Drying of the electrolyte: Naturally, the water contained in the electrolyte evaporates a little. Valve regulated lead acid (VRLA) batteries promote its recondensation, which reduces the need for additional distilled water (unlike open batteries). But, once the battery is charged, a supply of current initiates the electrolysis of the water with the formation of oxygen and hydrogen gas. In a VRLA battery, beyond a certain pressure, safety valves let the water escape permanently. This is problematic because topping up with distilled water is not possible.
How to avoid it? • Avoid overloads: check the data sheets to ensure that the load currents and durations are not too high. • Avoid high temperatures: ventilate or insulate the battery room, leave space between each battery.
• Sulfation: During discharge, crystals of lead sulfate (PbSO4) form on the positive and negative electrodes. If the battery remains discharged for a long time, these lead sulphate crystals grow and harden irreversibly. This reduces the conductivity of the electrodes, causes the battery to lose capacity and can cause short circuits.
How to limit it? • Avoid prolonged undercharging: never store a discharged battery. • Avoid incomplete charges: charge your batteries to 100% at least once a week.
• Freezing of Electrolyte: When a battery is discharged, the electrolyte is mostly water. At low temperatures, it can freeze and irreparably damage the battery.
How to avoid it? • Avoid too short daily journeys by car in winter. • In cold climates, increase the specific gravity of the electrolyte if the battery is open (acid/water = 1.29-1.3 g/cm3). • Switch to AGM or Gel batteries.
Corrosion of the battery terminals: Following acid splashes, acid vapours, or simply galvanic corrosion (two metals brought into contact), lead oxide deposits may form on the battery terminals. This can cause electrical conductivity problems.
How to avoid it? • Lubricate the connectors with petroleum jelly or an anti-corrosion grease suitable for batteries • Brush and clean the terminals if traces of corrosion appear.
• Fusion of the battery terminals: If the connector is loose on the terminal, the electrical contact resistance will increase. When a high current passes, the terminals can melt by the Joule effect (the conversion of electric energy into heat energy by resistance in a circuit). This can lead to fires.
How to avoid it? • Comply with the tightening torques in N.m given by the battery manufacturers. • Regularly check the correct tightening, especially if the batteries are subject to vibrations (for example, golf cars, trailers, etc.).
Étape 5 - Summary of good practices to adopt with lead acid batteries
- Matériel: Bien choisir sa batterie en fonction de l’usage recherché.
Ne jamais mélanger des batteries neuves et usagées.
Ne jamais mélanger des batteries de technologies différentes.
Installer correctement et solidement le câblage de votre parc batterie pour éviter les incendies.
Vérifier régulièrement les connectiques si celles-ci sont soumises à des vibrations.
- Détection et prévention des décharges profondes: La durée de vie d’une batterie est en relation directe avec la profondeur de décharge DoD. Il est donc très important d’empêcher toute décharge à plus de 50% !
- Comment connaitre le niveau de charge (SoC)?
- La mesure du voltage ne suffit pas. Trop de facteurs différents agissent sur la tension de batterie.
- Il faut utiliser un moniteur batterie. Il calcule la tension mais aussi les courants de charge et de décharge. Cela permet de calculer l'état de charge en direct.
- Comment éviter les décharges profondes ?
- L’idée est de contrôler le niveau de charge (SoC) et de déconnecter les charges de consommation dès que celui-ci passe sous un niveau établi.
- Utiliser un protecteur de batterie/Battery Protect ou un régulateur de charge solaire paramétrable, pour les équipements en courant continu DC.
- Utiliser le relais à contact sec de votre moniteur batterie s'il en est équipé.
- Paramétrer le seuil de basse tension batterie sur votre onduleur pour les équipements en courant alternatif AC (bien lire la notice).
- Comment connaitre le niveau de charge (SoC)?
- Faire attention à la température: Ce facteur a une influence très importante sur la durée de vie des batteries ! Il est très important de garder les batteries à des températures « fraiches » , environ 20°C.
- Local technique : Choisissez toujours la pièce ou l’endroit le plus frais. Ne laissez jamais les batteries exposées au soleil direct. Si ce lieu est encore trop chaud, il faudra très sérieusement envisager une ventilation rafraichissante du local ou du container batterie.
- Aération et ventilation : Toujours garder de l’espace entre les batteries (environ 5 cm), ne pas les mettre les unes contre les autres. Si les batteries sont à l’intérieur d’un coffre à batterie ou dans une armoire, il doit y exister une circulation d’air.
- Compensation de température: Lorsque la température dépasse les 30°C ou est inférieure à 10°C durant une longue période, il est nécessaire de modifier la tension de recharge.
- Batterie non utilisée – Autodécharge: Quand une batterie n’est pas utilisée, elle se décharge lentement. Ce phénomène dépend du type de batterie et de la température.
- Une batterie ouverte non utilisée doit rechargée tous les 4 mois à température ambiante (entre 10-25°C).
- Une batterie ouverte non utilisée doit être maintenue chargée en permanence par des température inférieure à 0°C.
- Les batteries étanches pourront être laissées jusqu’à 6 à 8 mois sans recharge par température ambiante.
- Quand un système contenant des batteries (camping car, voiture, etc) n’est pas utilisé pendant une longue période, débranchez les batteries pour éviter les courants de fuites.
- Tensions correctes de charge: Ne jamais recharger les batteries avec une tension supérieure à celle préconisée dans la fiche technique du fabriquant. Utiliser un chargeur ayant au moins 3 étapes de charge (Bulk, Absorption, Float).
- Courant correct de charge / décharge : Il est préconisé de ne jamais charger ou recharger des batteries plomb à plus de 0,2C, c'est-à-dire 20% de la capacité du parc batterie (ex: 20A pour un parc batterie de 100Ah).
Étape 6 - Désulfatation / Régénération de batteries au plomb
Durant la décharge, du sulfate de plomb (PbSO4) se forme sur les électrodes positives et négatives. Si la batterie reste déchargée, ce sulfate de plomb cristallise et durcit. Une fois cristallisé, il ne peut plus se transformer en acide sulfurique lors du chargement de la batterie. Cela fait chuter la capacité de la batterie: "elle ne tient plus la charge"
La régénération de batterie est un processus qui consiste à envoyer des impulsions électriques de forte intensité (300-400A) à une fréquence donnée, basée sur la fréquence de résonance propre de la batterie. Celle-ci est calculée automatiquement par la machine et évolue au cours du temps. Ces impulsions viennent briser la couche cristalline formée par le sulfate de plomb amorphe et permettent la redilution de celui-ci dans l'acide sulfurique.
Taux de succès: La sulfatation n'étant pas le seul phénomène de dégradation d'une batterie, toutes ne pourront pas être régénérées par désulfatation.
- Sur les batteries à électrodes tubulaires, le taux de succès est d'environ 90% (source: BeEnergy)
- Sur les batteries de démarrage, le taux de succès est d'environ 30%. (source: BeEnergy)
Durée du procédé: Ce procédé peut durer de quelques heures pour une batterie de démarrage à plusieurs jours pour des batteries de traction.
[Recherches à poursuivre]
Notes et références
Document rédigé par Guénolé Conrad avec l'aide de Loup Girier, Wiam Razi, Elliot Harant et Pascal Criquioche dans le cadre du projet Scholar Grid. Un projet à l'initiative de la Fondation Schneider Electric avec le support technique d'Energie Sans Frontières, Atelier 21 et du Low-tech Lab
- Document Victron Energy, Traduit de: ”Optimiser la vie des batteries plomb - Leçon V02 Bis.docx” de Margriet Leeftink, par Jacques Noël
- Bon résumé sur l'Installation des batteries au plomb, Site web "Batterie-solaire.com"
- Bon résumé sur l'Entretien des batteries au plomb, Site web "Batterie-solaire.com"
- Rapport "Etat de l'Art des Technologies de Désulfatation des Accumulateurs à Plomb" de l'ADEME - 2011
- Vidéo "Batterie Liquide, AGM, GEL, que choisir?" de la chaîne Youtube de Guillaume Piton - La Watterie
Published

 Français
Français English
English Deutsch
Deutsch Español
Español Italiano
Italiano Português
Português