(Page créée avec « These batteries are used in emergency power supplies, in particular for computer or telecommunication systems. They are designed so as to be constantly recharged and to be... ») |
|||
| (114 révisions intermédiaires par 2 utilisateurs non affichées) | |||
| Ligne 3 : | Ligne 3 : | ||
|Main_Picture_annotation={"version":"3.5.0","objects":[{"type":"image","version":"3.5.0","originX":"left","originY":"top","left":-20,"top":47,"width":470,"height":264,"fill":"rgb(0,0,0)","stroke":null,"strokeWidth":0,"strokeDashArray":null,"strokeLineCap":"butt","strokeDashOffset":0,"strokeLineJoin":"miter","strokeMiterLimit":4,"scaleX":1.35,"scaleY":1.35,"angle":0,"flipX":false,"flipY":false,"opacity":1,"shadow":null,"visible":true,"clipTo":null,"backgroundColor":"","fillRule":"nonzero","paintFirst":"fill","globalCompositeOperation":"source-over","transformMatrix":null,"skewX":0,"skewY":0,"crossOrigin":"","cropX":0,"cropY":0,"src":"https://wiki.lowtechlab.org/images/a/ab/Fonctionnement_entretien_et_r_g_n_ration_de_batteries_au_plomb_batterie-solaire-2v-12v-24v.jpg","filters":[]}],"height":449.59785522788206,"width":600} | |Main_Picture_annotation={"version":"3.5.0","objects":[{"type":"image","version":"3.5.0","originX":"left","originY":"top","left":-20,"top":47,"width":470,"height":264,"fill":"rgb(0,0,0)","stroke":null,"strokeWidth":0,"strokeDashArray":null,"strokeLineCap":"butt","strokeDashOffset":0,"strokeLineJoin":"miter","strokeMiterLimit":4,"scaleX":1.35,"scaleY":1.35,"angle":0,"flipX":false,"flipY":false,"opacity":1,"shadow":null,"visible":true,"clipTo":null,"backgroundColor":"","fillRule":"nonzero","paintFirst":"fill","globalCompositeOperation":"source-over","transformMatrix":null,"skewX":0,"skewY":0,"crossOrigin":"","cropX":0,"cropY":0,"src":"https://wiki.lowtechlab.org/images/a/ab/Fonctionnement_entretien_et_r_g_n_ration_de_batteries_au_plomb_batterie-solaire-2v-12v-24v.jpg","filters":[]}],"height":449.59785522788206,"width":600} | ||
|Licences=Attribution (CC BY) | |Licences=Attribution (CC BY) | ||
| − | |Description=Batteries are | + | |Description=Batteries are essential and expensive elements in off-grid installations. However, their operation and maintenance are not well known/not well understood by the general public. |
This tutorial, therefore, has several objectives: | This tutorial, therefore, has several objectives: | ||
* Present/Explain how a lead-acid battery works. | * Present/Explain how a lead-acid battery works. | ||
| − | *Present/Explain the different types of lead acid batteries | + | *Present/Explain the different types of lead acid batteries. |
* Present/Explain the major causes of degradation of lead batteries. | * Present/Explain the major causes of degradation of lead batteries. | ||
* Present/Explain the rules for the use and maintenance of lead batteries. | * Present/Explain the rules for the use and maintenance of lead batteries. | ||
| Ligne 27 : | Ligne 27 : | ||
| − | The damage will result in reduced battery life and, in some cases, there could be irreparable deterioration of batteries. Batteries will last longer when used properly, and so their replacement will be less frequent. In the long run, one can make considerable savings. Another interesting aspect is that the conversion system will be more efficient if the batteries are in a good condition. The better the batteries’ condition, the more efficient the installation will be. | + | The damage will result in reduced battery life and, in some cases, there could be irreparable deterioration of batteries. Batteries will last longer when used properly, and so their replacement will be less frequent. '''In the long run, one can make considerable savings'''. Another interesting aspect is that the conversion system will be more efficient if the batteries are in a good condition. The better the batteries’ condition, the more '''efficient''' the installation will be. |
| Ligne 37 : | Ligne 37 : | ||
}} | }} | ||
{{Tuto Step | {{Tuto Step | ||
| − | |Step_Title= | + | |Step_Title=Composition of a lead-acid battery |
| − | |Step_Content= | + | |Step_Content=*A lead battery is made up of 'a set of cells'. The nominal voltage of an accumulator/cell is approximately 2.1 V, and so a 12-V battery consists of six accumulator/cell mounted in series and connected by welded lead. (A series of cells connected in series, or parallel is called module) The cells are fitted/packed in a plastic container and sealed with a lid. |
| − | + | *Each cell comprises pairs of 'positive and negative electrodes' (plates) connected in parallel, with a separator in between each pair. | |
| − | + | *The 'separators' are generally rectangular porous sheets, inserted between the positive plates and the negative plates, and have the following important characteristics: | |
| − | + | **they serve/act as perfect electrical insulators. | |
| − | + | **they are highly permeable to ions carrying electrical charges. | |
| − | + | **they have excellent resistance to sulfuric acid, | |
| − | + | *The '''electrodes''' are composed of a '''grid''' on which is deposited a porous active material: '''lead (Pb)''' on the '''negative electrode''' and '''lead dioxide''' (PbO2) on the '''positive electrode'''. The grid collects the current and also serves as a mechanical support for the active material. | |
| − | + | *The '''electrolyte''' is a '''dilute solution of sulfuric acid''' in which the electrodes are immersed. It can be in liquid, gel or absorbed form in fiberglass felts, depending on the type of battery. | |
|Step_Picture_00=Fonctionnement__entretien_et_r_g_n_ration_de_batteries_au_plomb_Sch_ma_batteries.JPG | |Step_Picture_00=Fonctionnement__entretien_et_r_g_n_ration_de_batteries_au_plomb_Sch_ma_batteries.JPG | ||
|Step_Picture_01=Fonctionnement__entretien_et_r_g_n_ration_de_batteries_au_plomb_capture-decran-2020-07-06-a-08.54.22.png | |Step_Picture_01=Fonctionnement__entretien_et_r_g_n_ration_de_batteries_au_plomb_capture-decran-2020-07-06-a-08.54.22.png | ||
| Ligne 58 : | Ligne 58 : | ||
Reaction during discharge: During discharge, the following chemical reaction takes place: | Reaction during discharge: During discharge, the following chemical reaction takes place: | ||
| − | + | [https://fr.wikipedia.org/wiki/Dioxyde_de_plomb PbO<sub>2 sol</sub>] + [https://fr.wikipedia.org/wiki/Plomb Pb<sub> sol</sub>] + 2 [https://fr.wikipedia.org/wiki/Hydrog%C3%A9nosulfate HSO<sub>4</sub><sup>−</sup><sub>aq</sub>] + 2 [https://fr.wikipedia.org/wiki/Proton H<sup>+</sup><sub>aq</sub>] ⟶ 2 [https://fr.wikipedia.org/wiki/Sulfate_de_plomb(II) PbSO<sub>4 sol</sub>] + 2 [https://fr.wikipedia.org/wiki/Eau H<sub>2</sub>O<sub> liq</sub>] | |
| − | + | **The positive (+) electrode which is '''lead dioxide''' converts into '''lead sulphate''' crystals. | |
| − | + | **The negative electrode (-) which is made of '''lead''' also changes into '''lead sulphate''' crystals. | |
| − | + | **The '''electrolyte''' bath in which the reactions take place is largely transformed into water (H2O). | |
| − | |||
| − | |||
| − | Reaction during Charge | + | *'''Reaction during Charge :''' When charging, the reverse chemical reaction takes place: |
| − | |||
| − | When charging, the reverse chemical reaction takes place: | ||
| − | |||
[https://fr.wikipedia.org/wiki/Sulfate_de_plomb(II) 2PbSO<sub>4 sol</sub>] + 2 [https://fr.wikipedia.org/wiki/Eau H<sub>2</sub>O<sub> liq</sub>] ⟶ [https://fr.wikipedia.org/wiki/Plomb Pb<sub> sol</sub>] + [https://fr.wikipedia.org/wiki/Dioxyde_de_plomb PbO<sub>2 sol</sub>] + 2 [https://fr.wikipedia.org/wiki/Hydrog%C3%A9nosulfate HSO<sub>4</sub><sup>−</sup><sub>aq</sub>] + 2 [https://fr.wikipedia.org/wiki/Proton H<sup>+</sup><sub>aq</sub>]. | [https://fr.wikipedia.org/wiki/Sulfate_de_plomb(II) 2PbSO<sub>4 sol</sub>] + 2 [https://fr.wikipedia.org/wiki/Eau H<sub>2</sub>O<sub> liq</sub>] ⟶ [https://fr.wikipedia.org/wiki/Plomb Pb<sub> sol</sub>] + [https://fr.wikipedia.org/wiki/Dioxyde_de_plomb PbO<sub>2 sol</sub>] + 2 [https://fr.wikipedia.org/wiki/Hydrog%C3%A9nosulfate HSO<sub>4</sub><sup>−</sup><sub>aq</sub>] + 2 [https://fr.wikipedia.org/wiki/Proton H<sup>+</sup><sub>aq</sub>]. | ||
| Ligne 76 : | Ligne 71 : | ||
<br /> | <br /> | ||
| − | Lead sulphate crystals dissolve/(are broken down into) lead dioxide which is deposited on the (+) electrode and lead which is deposited on the (-) electrode. | + | **'''Lead sulphate crystals dissolve'''/(are broken down into) lead dioxide which is deposited on the (+) electrode and lead which is deposited on the (-) electrode. |
| − | + | **The electrolyte reverts to dilute sulfuric acid. | |
<br /> | <br /> | ||
| Ligne 83 : | Ligne 78 : | ||
}} | }} | ||
{{Tuto Step | {{Tuto Step | ||
| − | |Step_Title=The units of the batteries are indicated as abbreviations which are not always easy to understand. Here is a summary table of the units associated with the batteries: | + | |Step_Title=The characteristic units of the batteries |
| − | | | + | |Step_Content=The units of the batteries are indicated as abbreviations which are not always easy to understand. Here is a summary table of the units associated with the batteries : |
| − | Capacity (Ah) | + | {| class="wikitable" |
| − | Tension (V) | + | |+ |
| − | + | !Characteristic | |
| − | Energy (Wh) | + | !Definition |
| − | + | !Explanation | |
| − | Discharge rate, | + | |- |
| − | + | |Capacity (Ah) | |
| − | + | |The amount of current that a battery can store or release, usually specified in Ah for a given discharge rate. | |
| − | Cold Cracks Amps (CCA) | + | |A 10 Ah battery can produce 5 Amperes (A) for 2 hours (h). |
| − | + | |- | |
| − | SOC (State of Charge) | + | |Tension (V) |
| − | DOD (Depth of Discharge) | + | |Battery voltage level. It must be compatible with the connected devices. |
| − | Number of cycles For a battery, a cycle represents a discharge followed by a charge. The number of cycles of a battery depends on the depth of discharge or amount of electricity consumed. The higher the DOD, the lower the cycle life | + | |Lead-acid batteries are made up of units delivering 2.1 Volts (V) and connecting these units in series makes it possible to reach the generally desired voltage. For example, six units connected in series deliver 12 V. To create 24 V or 48 V systems, 12 V batteries are, in turn, connected in series. |
| − | + | |- | |
| + | |Energy (Wh) | ||
| + | |The product of multiplication of the capacity by the voltage. | ||
| + | |A 200Ah 24V battery will have an energy of 4800 Watts hour (Wh). | ||
| + | |- | ||
| + | |Discharge rate, C<sub>xx</sub> | ||
| + | |Expressed as a unit of C<sub>10</sub>, C<sub>20</sub>or C<sub>100</sub>, it indicates the capacity of a battery according to its rate of discharge. | ||
| + | |50Ah C<sub>20</sub>battery means a battery of 50Ah capacity with 20h discharge | ||
| + | C<sub>100</sub>battery: 90Ah (capacity of 90Ah with a discharge in 100h). | ||
| + | |- | ||
| + | |Cold Cracks Amps (CCA) | ||
| + | |This is the maximum extractable current from a battery over a short period when starting the engine, for example. | ||
| + | |CCA 420A 5 sec indication means the battery can deliver 420A for 5 sec. | ||
| + | |- | ||
| + | |SOC (State of Charge) | ||
| + | |State of charge of a battery, which indicates the amount of electricity remaining. | ||
| + | |SOC = 50 %: the battery’s charge is 50%. | ||
| + | |- | ||
| + | |DOD (Depth of Discharge) | ||
| + | |State of discharge of a battery, or the amount of electricity consumed. | ||
| + | |DOD + SOC = 100% . | ||
| + | |- | ||
| + | |Number of cycles | ||
| + | |For a battery, a cycle represents a discharge followed by a charge. The number of cycles of a battery depends on the depth of discharge or amount of electricity consumed. | ||
| + | |The higher the DOD, the lower the cycle life, the same battery can have. | ||
| − | 500 cycles at 80% DOD | + | *500 cycles at 80% DOD |
| − | + | *750 cycles at 50% DOD | |
| − | + | *1800 cycles at 30% DOD | |
<br /> | <br /> | ||
| Ligne 111 : | Ligne 130 : | ||
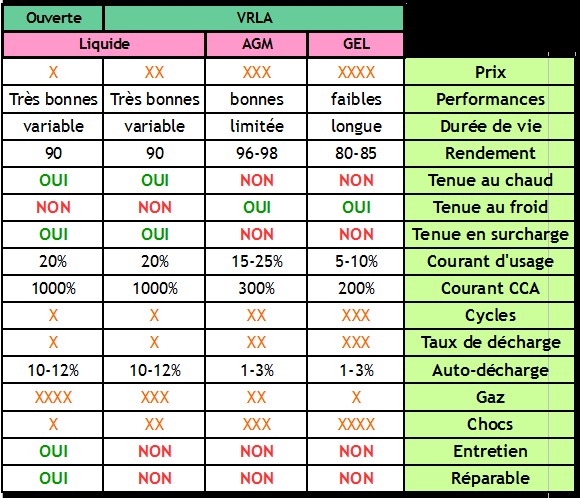
|Step_Content=There are several types of and technologies for lead batteries, each adapted to a particular use, environment and constraints. Understanding the differences is essential to choosing and maintaining your battery correctly. This part summarizes the main categories of lead acid batteries and their characteristics. | |Step_Content=There are several types of and technologies for lead batteries, each adapted to a particular use, environment and constraints. Understanding the differences is essential to choosing and maintaining your battery correctly. This part summarizes the main categories of lead acid batteries and their characteristics. | ||
| − | <br />{{Warning| | + | <br />{{Warning|Never mix batteries of different types. |
It is important to note that one should never mix batteries of different types. The following combinations are to be avoided: | It is important to note that one should never mix batteries of different types. The following combinations are to be avoided: | ||
| − | + | *Old and new batteries. | |
| − | + | *Different capacities. | |
| − | + | *Different battery types. | |
| − | + | *Different brands. | |
| − | + | *Different technologies or chemistry}}<br /> | |
| + | ====Batteries according to their use : ==== | ||
| − | + | *'''<u>Starter battery:</u>''' | |
| − | A starter battery is intended to provide high current for a very short time. It is designed to start an engine (for example a vehicle or a generator). Starter batteries are sometimes called "car battery", "truck battery" or "thin plate battery". | + | A starter battery is intended to provide high current for a very short time. It is designed to start an engine (for example a vehicle or a generator). Starter batteries are sometimes called "car battery", "truck battery" or "thin plate battery". |
| + | [https://www.youtube.com/watch?v=fYGvX-jkVtQ&t=282 View inside a starter battery .] | ||
| + | <br /> | ||
| + | {{Warning|Starter batteries are not made for cyclic use. They are designed only for high discharge currents of very short duration. Thus, they cannot be used in an electrical conversion system / photovoltaic installation. Even if it is tempting to use them because they are easily available at low cost, it will cause malfunctions finally.}} | ||
| − | + | *'''<u>Traction battery</u>''' | |
| − | + | The name of these batteries comes from their first use: powering the motor of electric vehicles such as forklifts. They are generally equipped with "thick or tubular plates" which allows them to '''withstand fairly deep discharges and have a long lifespan'''. They are well suited for use in solar photovoltaics. | |
| − | The | + | The '''batteries [https://www.batterie-solaire.com/batterie-OPZS OPzS] ('''liquid electrolyte''') and [https://www.batterie-solaire.com/batterie-opzv OPzV]''' (gel electrolyte) have almost the same characteristics as traction batteries. |
| − | + | <br /> | |
| − | < | + | *'''<u>Stationary battery</u>''' |
| − | + | These batteries are used in '''emergency power supplies''', in particular for computer or telecommunication systems. They are designed so as to be constantly recharged and to be discharged only infrequently. | |
| − | |||
| + | *'''<u>Solar battery / slow discharge</u>''' | ||
| − | + | These batteries are intended for use in photovoltaic solar installations. They are designed to withstand a high number of cycles (since they will be discharged every night and recharged every morning), and their depth of discharge is generally good but can vary greatly from one model to another. Service batteries have almost the same characteristics as solar batteries. | |
| − | + | <br />[https://www.youtube.com/watch?v=4tdPLAsTKNo View the inside a slow cycle/solar battery] | |
| − | <br />[https://www.youtube.com/watch?v=4tdPLAsTKNo | ||
| − | ==== | + | ====Batteries according to their technology / electrolyte==== |
| − | *'''<u> | + | *'''<u>Open battery</u>''' |
| − | + | An open battery is a battery with liquid electrolyte equipped with plugs allowing to fill it. Open batteries are not watertight: the liquid inside evaporates little by little, so it is necessary to check its level regularly and top it up if necessary with distilled water. | |
{| class="wikitable" | {| class="wikitable" | ||
|+ | |+ | ||
| − | ! | + | !Advantages |
| − | ! | + | !Disadvantages |
|- | |- | ||
| − | | | + | |Repairable |
| − | | | + | |Maintenance required |
|- | |- | ||
| − | | | + | |Delivers current in cold temperatures ( Their CCA rating indicates the battery has sufficient power to crank an engine in very cold temperatures) |
| − | | | + | |Risk of non-homogeneity of the electrolyte if little used = premature aging |
|- | |- | ||
| − | | | + | |Withstands overloads and overheating (one can add liquid if it evaporates) |
| − | | | + | |Release of hydrogen and, therefore, risk of explosion if environment not ventilated |
|- | |- | ||
| − | | | + | |Low cost |
| − | | | + | |Not conducive to cold, risk of electrolyte freezing |
|- | |- | ||
| | | | ||
| − | | | + | |Strong self-discharge (10-12% per month) if not used regularly. |
|- | |- | ||
| | | | ||
| − | | | + | |Leaks possible if there is tilting or shaking/vibrations |
|} | |} | ||
<br /> | <br /> | ||
| − | *'''<u> | + | *'''<u>Sealed, leak-proof liquid batteries</u>''' |
| − | + | A sealed battery is a liquid electrolyte battery equipped with a system to prevent the evaporation of the water contained in the electrolyte, by gas recombination. These batteries do not require maintenance, and are often called '''VRLA''' for "Valve Regulated Lead-Acid batteries". | |
{| class="wikitable" | {| class="wikitable" | ||
|+ | |+ | ||
| − | ! | + | !Advantages |
| − | ! | + | !Disadvantages |
|- | |- | ||
| − | | | + | |Reduces explosive gas production, water loss and leakage. |
| − | | | + | |Does not allow maintenance or control. |
|- | |- | ||
| − | | | + | |Requires less maintenance. |
| − | | | + | |Imposes a perfectly regulated load according to the temperature to avoid gas losses by excessive pressure. |
|} | |} | ||
<br /> | <br /> | ||
| − | *'''<u> | + | *'''<u>AGM batteries</u>''' |
| − | + | AGM batteries are a type of sealed/VRLA battery, in which the electrolyte is a liquid but it is held in place in a fiberglass blotter, and hence its name: Absorbed Glass Material. | |
| − | <br />[https://www.youtube.com/watch?v=MySMrdb2nwQ | + | <br />[https://www.youtube.com/watch?v=MySMrdb2nwQ View the inside of an AGM battery] |
{| class="wikitable" | {| class="wikitable" | ||
|+ | |+ | ||
| − | ! | + | !Advantages |
| − | ! | + | !Disadvantages |
|- | |- | ||
| − | | | + | |Maintenance-free with minimal release of gas |
| − | | | + | |They do not perform well in hot conditions (loss of electrolyte in the form of gas at higher temperatures) A temperature above 49°C (120°F) is very dangerous for the battery life. |
|- | |- | ||
| − | | | + | |They maintain the electrolyte homogeneity well. |
| − | | | + | |They are sensitive to overcharging and high voltages (loss of electrolyte in the form of gas) |
|- | |- | ||
| − | | | + | |Withstand colder temperatures well because of their homogeneous electrolyte (Since the electrolyte is held in the glass mat separators, it won't expand when frozen like it will in a flooded battery) |
| − | | | + | |They have limited shelf-life (as the acid concentration inside is higher than in others, which leads to faster battery degradation). |
|- | |- | ||
| − | | | + | |Allows high peak currents (CCA) to pass |
| | | | ||
|- | |- | ||
| − | | | + | |Shock-resistant (Vibration-resistant) (Because of the fibre glass mats are woven tightly and the plates are packed tightly, making them immune to vibrations) |
| | | | ||
|- | |- | ||
| − | | | + | |Low self-discharge (1-3% per month) |
| | | | ||
|} | |} | ||
| − | *'''<u> | + | *'''<u>Gel batteries</u>''' |
| − | + | Gel batteries are a type of sealed battery / VRLA. In a gel battery, the electrolyte is gelled by adding silicate. | |
| − | <br />[https://www.youtube.com/watch?v=d7pwE3u0kmo | + | <br />[https://www.youtube.com/watch?v=d7pwE3u0kmo View the inside of a gel battery] |
{| class="wikitable" | {| class="wikitable" | ||
|+ | |+ | ||
| − | ! | + | !Advantages |
| − | ! | + | !Disadvantages |
|- | |- | ||
| − | | | + | |Maintains the homogeneity of the electrolyte perfectly. |
| − | | | + | |Limited peak current. |
|- | |- | ||
| − | | | + | |Low self-discharge (1-3% per month). |
| − | | | + | |Slow charging and discharging. (charge current limited to 5-10% of capacity). |
|- | |- | ||
| − | | | + | |Resists shocks and vibrations well because everything is well maintained inside .|Cannot withstand high temperatures (loss of electrolyte in the form of gas - permanent effect). |
| − | | | ||
|- | |- | ||
| − | | | + | |Longer life span/shelf life. |
| − | | | + | |Sensitive to overload (loss of electrolyte in the form of gas). |
|- | |- | ||
| | | | ||
| − | | | + | |High cost |
|} | |} | ||
|Step_Picture_00=Fonctionnement__entretien_et_r_g_n_ration_de_batteries_au_plomb_bat_anim_118.bmp | |Step_Picture_00=Fonctionnement__entretien_et_r_g_n_ration_de_batteries_au_plomb_bat_anim_118.bmp | ||
| Ligne 248 : | Ligne 269 : | ||
}} | }} | ||
{{Tuto Step | {{Tuto Step | ||
| − | |Step_Title= | + | |Step_Title=Mechanisms of degradation of Lead Acid Batteries |
| − | |Step_Content=*'''Stratification | + | |Step_Content=*'''Stratification of the electrolyte''': In a wet electrolyte battery, if the electrolyte is not agitated, the sulfuric acid will flow down the trays./plates. Thus, the density of the electrolyte will slowly increase at the bottom of the batteries, while it will decrease at the top of the batteries. This stratification of the acid will result in non-homogeneous electrode discharge with accelerated corrosion at the bottom of the battery. |
| − | {{Idea| | + | |
| − | * | + | {{Idea|How to avoid it ? |
| − | * | + | *Use batteries regularly -- The electrolysis of water creates bubbles of oxygen which agitate the electrolyte. |
| − | + | *Periodically carry out an equalization charge -- This consists of charging the batteries with a low current, but with higher voltage than that generally applied to create greater bubbling. | |
| + | • Use gel or AGM batteries.}} | ||
| − | *'''Corrosion | + | *'''Corrosion of the positive electrodes''': The positive electrodes are sensitive to corrosion which occurs when not in use, but especially during charging, when the lead in the grid is transformed into lead oxide, which is not very conductive. If there is too much corrosion, the active materials gradually sink to the bottom of the accumulators, and electrodes disintegrate. The capacity of the battery decreases and the internal resistance increases until the battery becomes unusable. |
| − | {{Idea| | + | {{Idea|How to limit it? |
| − | * | + | *Avoid overloads: check the data sheets so that the load currents and duration are not too high. |
| − | * | + | *Avoid high temperatures: ventilate or insulate the battery room, leave a space between each battery.}}<br /> |
| − | *''' | + | *'''Loss of active material''': During charge and discharge cycles, the positive and negative plates undergo strong mechanical stresses (high currents, induced magnetic fields). The plates gradually disintegrate and the active material accumulates at the bottom of the battery. This "mud" can cause short circuits between two plates. |
| − | {{Idea| | + | {{Idea|How to avoid it ? |
| − | * | + | *Avoid deep discharges. |
| − | * | + | *Avoid rapid discharges: If the battery is discharged very quickly, the mechanical stresses do not have time to accommodate themselves and disintegration is faster. |
| − | * | + | *Choose batteries with thick or tubular electrodes.}} |
| − | *''' | + | *'''Drying of the electrolyte:''' Naturally, the water contained in the electrolyte evaporates a little. Valve regulated lead acid (VRLA) batteries promote its recondensation, which reduces the need for additional distilled water (unlike open batteries). But, once the battery is charged, a supply of current initiates the electrolysis of the water with the formation of oxygen and hydrogen gas. In a VRLA battery, beyond a certain pressure, safety valves let the water escape permanently. This is problematic because topping up with distilled water is not possible. |
| − | {{Idea| | + | {{Idea|How to avoid it ? |
| − | * | + | *Avoid overloads: check the data sheets to ensure that the load currents and durations are not too high. |
| − | * | + | *Avoid high temperatures: ventilate or insulate the battery room, leave space between each battery.}} |
| − | *''' | + | *'''Sulfation''': During discharge, crystals of lead sulfate (PbSO4) form on the positive and negative electrodes. If the battery remains discharged for a long time, these lead sulphate crystals grow and harden irreversibly. This reduces the conductivity of the electrodes, causes the battery to lose capacity and can cause short circuits. |
| − | {{Idea| | + | {{Idea|How to limit it? |
| − | * | + | *Avoid prolonged undercharging: never store a discharged battery. |
| − | * | + | *Avoid incomplete charges: charge your batteries to 100% at least once a week.}} |
| − | ''' | + | '''Freezing of Electrolyte''': When a battery is discharged, the electrolyte is mostly water. At low temperatures, it can freeze and irreparably damage the battery. |
| − | {{Idea|1= | + | {{Idea|1=How to avoid it? |
| − | * | + | *Avoid too short daily journeys by car in winter. |
| − | * | + | *In cold climates, increase the specific gravity of the electrolyte if the battery is open (acid/water = 1.29-1.3 g/cm3). |
| − | * | + | *Switch to AGM or Gel batteries.}} |
| − | + | ''' Corrosion of the battery terminals''' : Following acid splashes, acid vapours, or simply galvanic corrosion (two metals brought into contact), lead oxide deposits may form on the battery terminals. This can cause electrical conductivity problems. | |
| − | {{Idea| | + | {{Idea|How to avoid it? |
| − | * | + | *Lubricate the connectors with petroleum jelly or an anti-corrosion grease suitable for batteries |
| − | * | + | *Brush and clean the terminals if traces of corrosion appear.}} |
| − | *'''Fusion | + | *'''Fusion of the battery terminals''': If the connector is loose on the terminal, the electrical contact resistance will increase. When a high current passes, the terminals can melt by the Joule effect (the conversion of electric energy into heat energy by resistance in a circuit). This can lead to fires. |
| − | {{Idea| | + | {{Idea|How to avoid it ? |
| − | * | + | *Comply with the tightening torques in N.m given by the battery manufacturers. |
| − | * | + | *Regularly check the correct tightening, especially if the batteries are subject to vibrations (for example, golf cars, trailers, etc.).}}<br /> |
|Step_Picture_00=Fonctionnement__entretien_et_r_g_n_ration_de_batteries_au_plomb_Tableau_causes_d_gradation_batteries_plomb_goodyy.JPG | |Step_Picture_00=Fonctionnement__entretien_et_r_g_n_ration_de_batteries_au_plomb_Tableau_causes_d_gradation_batteries_plomb_goodyy.JPG | ||
}} | }} | ||
{{Tuto Step | {{Tuto Step | ||
| − | |Step_Title= | + | |Step_Title=Summary of good practices to adopt with lead acid batteries |
| − | |Step_Content= | + | |Step_Content='''Equipment''': Choose your battery carefully according to the intended use. <br />Never mix new and used batteries. <br />Never mix batteries of different technologies. <br />Correctly and solidly install the wiring of your battery bank to avoid fires. <br />Regularly check the connectors if they are subject to vibrations. |
<br /> | <br /> | ||
| − | *''' | + | *'''Detection and Prevention of Deep Discharge''': Battery life is directly related to DoD or depth of discharge. It is, therefore, very important to prevent any discharge over 50% !''' <br /> |
| − | **<u> | + | **<u>How to know the level of charge (SoC)?</u> |
| − | *** | + | ***Simply measuring the voltage does not suffice as several factors affect the battery voltage. |
| − | *** | + | ***A [https://www.victronenergy.fr/battery-monitors battery monitor] must be used. It calculates not only the voltage but also the charge and discharge currents, which allows the state of charge to be calculated in real time. |
| − | **<u> | + | **<u>How to avoid deep discharges?</u> |
| − | *** | + | ***The idea is to control the level of charge (SoC) and to disconnect the consumption loads as soon as they fall below a certain level. |
| − | *** | + | ***Use a battery protector/[https://www.victronenergy.fr/battery_protect/battery-protect Battery Protect] or a configurable solar charge regulator, for direct current (DC) equipment. |
| − | *** | + | ***Use the dry contact relay (voltage-free relay) of your battery monitor if it is equipped with one. |
| − | *** | + | ***Set the low battery voltage threshold on your inverter for alternating current (AC) equipment (read the instructions carefully). |
| − | *''' | + | *'''Pay attention to the temperature: ''': This factor has a very important influence on the life of the batteries. It is very important to keep the batteries at “cool” temperatures, around 20°C. |
| − | **<u> | + | **<u>Technical roome</u> : Always choose the coolest room or location. Never leave batteries exposed to direct sunlight. If the place is still too hot, one should consider cooling ventilation of the room or the battery container. |
| − | **<u> | + | **<u>Aeration and ventilation</u> : Always keep space between the batteries (about 5 cm), do not put them against each other. If the batteries are inside a battery box or in a cabinet, there must be air circulation. |
| − | **<u> | + | **<u>Temperature compensation:</u> When the temperature exceeds 30°C or is lower than 10°C for a long time, it is necessary to change the charging voltage. |
<br /> | <br /> | ||
| − | + | Battery not in use – Self-discharge: When a battery is not in use, it slowly discharges. This phenomenon depends on the type of battery and the temperature. | |
| − | + | o An unused open battery must be recharged every four months at room temperature (between 10-25°C). | |
| − | + | o An unused open battery must be kept permanently charged in temperatures below 0°C. | |
| − | + | o Sealed batteries can be left for up to 6 to 8 months without recharging at ambient temperature. | |
| − | + | o When a system containing batteries (RV, car, etc.) is not used for a long period, disconnect the batteries to avoid leakage currents. | |
<br /> | <br /> | ||
| − | *''' | + | *'''Correct charging voltages''': Never recharge the batteries with a voltage higher than that recommended in the manufacturer's data sheet. Use a charger with at least 3 charge stages (Bulk, Absorption, Float). |
<br /> | <br /> | ||
| − | *''' | + | *'''Correct charge/discharge current''': '''It is recommended '''never to charge or recharge''' lead batteries at '''more than 0.2C''', i.e. 20% of the capacity of the battery bank (ex: 20A for a battery bank of 100Ah). |
| − | {{Idea|1= | + | {{Idea|1=When sizing a photovoltaic installation, make sure that the maximum output current is less than 20% of the battery capacity. Let: Imax (A) = Pmax (W) / Ubat (V) < 0.2C}} <br /> <br /> |
<br /> | <br /> | ||
}} | }} | ||
{{Tuto Step | {{Tuto Step | ||
| − | |Step_Title= | + | |Step_Title=Desulphation/Regeneration of lead acid batteries |
| − | |Step_Content= | + | |Step_Content=During discharge, lead sulphate (PbSO4) forms on the positive and negative electrodes. If the battery remains discharged, this lead sulphate crystallizes and hardens. Once crystallized, it can no longer turn into sulfuric acid when charging the battery. This causes the battery capacity to drop: "it no longer holds a charge" it is a weak battery/it is a dead battery. |
| − | + | '''Battery regeneration''' is a process of sending high-intensity electrical pulses (300-400A) at a given frequency, based on the battery's resonant frequency. This is calculated automatically by the machine and evolves over time. These impulses break down the crystalline layer formed by the amorphous lead sulphate deposited on the electrodes and convert it back to sulphuric acid. The plates are reconstituted and the battery returns to its original condition. | |
| − | ''' | + | '''Success rate''': Since sulphation is not the only phenomenon underlying battery degradation, not all of them can be regenerated by desulphation. |
| − | * | + | *For batteries with tubular electrodes, the success rate is around 90% (source: BeEnergy) |
| − | * | + | *For starter batteries, the success rate is around 30%. (source: BeEnergy) |
| − | ''' | + | '''Duration of the process''': This process can last from a few hours for a starter battery to several days for traction batteries. |
| − | + | Research to follow (For Further Reading) | |
<br /> | <br /> | ||
}} | }} | ||
{{Notes | {{Notes | ||
| − | |Notes=Document | + | |Notes=Document by Guénolé Conrad with the help of Loup Girier, Wiam Razi, Elliot Harant and Pascal Criquioche within the framework of the Scholar Grid project. A project initiated by the [https://www.se.com/fr/fr/about-us/sustainability/foundation/ Schneider Electric Foundation] with the technical support of [http://www.energies-sans-frontieres.org/ Energie Sans Frontières], [https://www.atelier21.org Atelier 21] and the [https://lowtechlab.org/en Low-tech Lab]<br /> |
| − | *[https://www.victronenergy.fr/upload/documents/Optimiser-la-vie-des-batteries-plomb-Le%C3%A7on-V02-Bis.pdf Document Victron Energy], | + | *[https://www.victronenergy.fr/upload/documents/Optimiser-la-vie-des-batteries-plomb-Le%C3%A7on-V02-Bis.pdf Document Victron Energy], Translated from: “Optimizing the life of lead batteries - Lesson V02 Bis.docx” by Margriet Leeftink, by Jacques Noël” |
| − | * | + | *Summary on the Installation of lead batteries: Website: Batterie-solaire.com |
| − | * | + | *Summary on the Maintenance of lead batteries: Website: Batterie-solaire.com |
| − | *[https://librairie.ademe.fr/recherche-et-innovation/3524-etat-de-l-art-des-technologies-de-desulfatation-des-accumulateurs-au-plomb.html?search_query=2022&results=1384 | + | *[https://librairie.ademe.fr/recherche-et-innovation/3524-etat-de-l-art-des-technologies-de-desulfatation-des-accumulateurs-au-plomb.html?search_query=2022&results=1384 Report] "State of the Art of Desulphation Technologies for Lead-Acid Accumulators" by ADEME - 2011 |
| − | *[https://www.youtube.com/watch?v=ryqKYLWlubE | + | *[https://www.youtube.com/watch?v=ryqKYLWlubE Video] "Liquid battery, AGM, GEL, what to choose?" from the Youtube channel of Guillaume Piton - La Watterie<br /> |
}} | }} | ||
{{PageLang | {{PageLang | ||
Version actuelle datée du 30 mai 2023 à 10:56
Description
Batteries are essential and expensive elements in off-grid installations. However, their operation and maintenance are not well known/not well understood by the general public. This tutorial, therefore, has several objectives:
- Present/Explain how a lead-acid battery works.
- Present/Explain the different types of lead acid batteries.
- Present/Explain the major causes of degradation of lead batteries.
- Present/Explain the rules for the use and maintenance of lead batteries.
- Introduce the process of desulfation (or regeneration) of lead batteries.
- Present/Explain how a lead-acid battery works.
- Present/Explain the different types of lead acid batteries.
- Present/Explain the major causes of degradation of lead batteries.
- Present/Explain the rules for the use and maintenance of lead batteries.
- Introduce the process of desulfation (or regeneration) of lead batteries.
Sommaire
Sommaire
- 1 Description
- 2 Sommaire
- 3 Introduction
- 4 Étape 1 - Composition of a lead-acid battery
- 5 Étape 2 - Operation of a lead acid battery
- 6 Étape 3 - The characteristic units of the batteries
- 7 Étape 4 - Different Types of Batteries for Different Uses
- 8 Étape 5 - Mechanisms of degradation of Lead Acid Batteries
- 9 Étape 6 - Summary of good practices to adopt with lead acid batteries
- 10 Étape 7 - Desulphation/Regeneration of lead acid batteries
- 11 Notes et références
- 12 Commentaires
Introduction
Batteries are often the most expensive and most fragile constituents of an electrical conversion system. Hence, it is important to take care of them through proper use and monitoring.
Lead acid batteries are very fragile. They are sensitive to overcharging, partial charging, deep discharges, excessively rapid charges, and to temperatures above 20°C. All these factors can lead to premature aging, mainly due to a combination of lack of technical knowledge, poorly- sized systems and erroneous use by a person. If one does not control these factors, the batteries will quickly be damaged.
The damage will result in reduced battery life and, in some cases, there could be irreparable deterioration of batteries. Batteries will last longer when used properly, and so their replacement will be less frequent. In the long run, one can make considerable savings. Another interesting aspect is that the conversion system will be more efficient if the batteries are in a good condition. The better the batteries’ condition, the more efficient the installation will be.
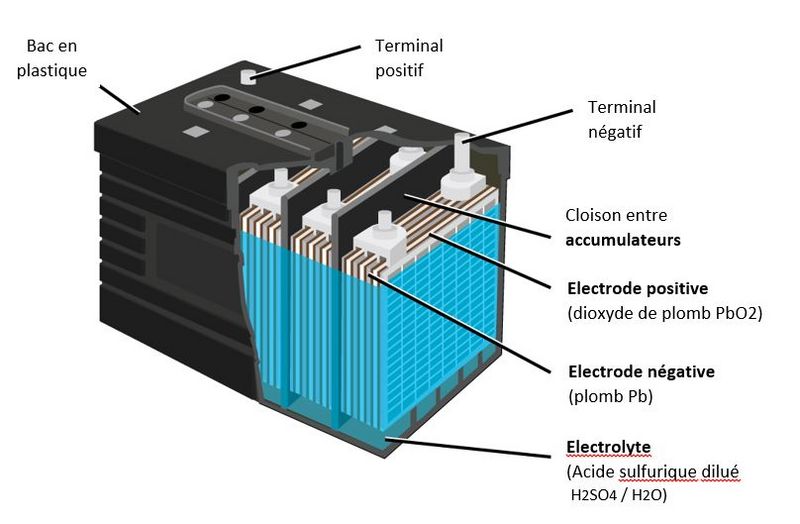
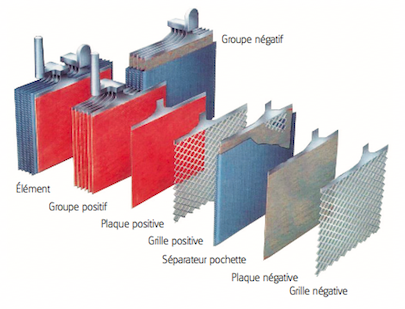
Étape 1 - Composition of a lead-acid battery
- A lead battery is made up of 'a set of cells'. The nominal voltage of an accumulator/cell is approximately 2.1 V, and so a 12-V battery consists of six accumulator/cell mounted in series and connected by welded lead. (A series of cells connected in series, or parallel is called module) The cells are fitted/packed in a plastic container and sealed with a lid.
- Each cell comprises pairs of 'positive and negative electrodes' (plates) connected in parallel, with a separator in between each pair.
- The 'separators' are generally rectangular porous sheets, inserted between the positive plates and the negative plates, and have the following important characteristics:
- they serve/act as perfect electrical insulators.
- they are highly permeable to ions carrying electrical charges.
- they have excellent resistance to sulfuric acid,
- The electrodes are composed of a grid on which is deposited a porous active material: lead (Pb) on the negative electrode and lead dioxide (PbO2) on the positive electrode. The grid collects the current and also serves as a mechanical support for the active material.
- The electrolyte is a dilute solution of sulfuric acid in which the electrodes are immersed. It can be in liquid, gel or absorbed form in fiberglass felts, depending on the type of battery.
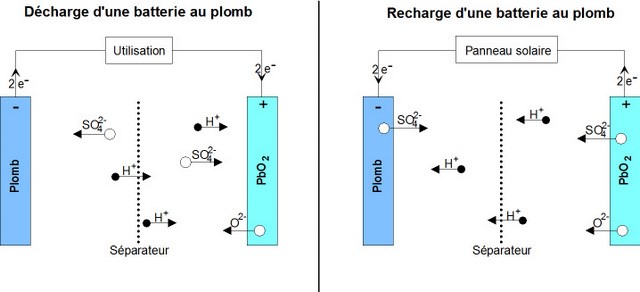
Étape 2 - Operation of a lead acid battery
To understand the causes of battery failure, it is important to understand the chemical reactions at work inside it.
Reaction during discharge: During discharge, the following chemical reaction takes place:
PbO2 sol + Pb sol + 2 HSO4−aq + 2 H+aq ⟶ 2 PbSO4 sol + 2 H2O liq
- The positive (+) electrode which is lead dioxide converts into lead sulphate crystals.
- The negative electrode (-) which is made of lead also changes into lead sulphate crystals.
- The electrolyte bath in which the reactions take place is largely transformed into water (H2O).
- Reaction during Charge : When charging, the reverse chemical reaction takes place:
2PbSO4 sol + 2 H2O liq ⟶ Pb sol + PbO2 sol + 2 HSO4−aq + 2 H+aq.
- Lead sulphate crystals dissolve/(are broken down into) lead dioxide which is deposited on the (+) electrode and lead which is deposited on the (-) electrode.
- The electrolyte reverts to dilute sulfuric acid.
Étape 3 - The characteristic units of the batteries
The units of the batteries are indicated as abbreviations which are not always easy to understand. Here is a summary table of the units associated with the batteries :
| Characteristic | Definition | Explanation |
|---|---|---|
| Capacity (Ah) | The amount of current that a battery can store or release, usually specified in Ah for a given discharge rate. | A 10 Ah battery can produce 5 Amperes (A) for 2 hours (h). |
| Tension (V) | Battery voltage level. It must be compatible with the connected devices. | Lead-acid batteries are made up of units delivering 2.1 Volts (V) and connecting these units in series makes it possible to reach the generally desired voltage. For example, six units connected in series deliver 12 V. To create 24 V or 48 V systems, 12 V batteries are, in turn, connected in series. |
| Energy (Wh) | The product of multiplication of the capacity by the voltage. | A 200Ah 24V battery will have an energy of 4800 Watts hour (Wh). |
| Discharge rate, Cxx | Expressed as a unit of C10, C20or C100, it indicates the capacity of a battery according to its rate of discharge. | 50Ah C20battery means a battery of 50Ah capacity with 20h discharge
C100battery: 90Ah (capacity of 90Ah with a discharge in 100h). |
| Cold Cracks Amps (CCA) | This is the maximum extractable current from a battery over a short period when starting the engine, for example. | CCA 420A 5 sec indication means the battery can deliver 420A for 5 sec. |
| SOC (State of Charge) | State of charge of a battery, which indicates the amount of electricity remaining. | SOC = 50 %: the battery’s charge is 50%. |
| DOD (Depth of Discharge) | State of discharge of a battery, or the amount of electricity consumed. | DOD + SOC = 100% . |
| Number of cycles | For a battery, a cycle represents a discharge followed by a charge. The number of cycles of a battery depends on the depth of discharge or amount of electricity consumed. | The higher the DOD, the lower the cycle life, the same battery can have.
|
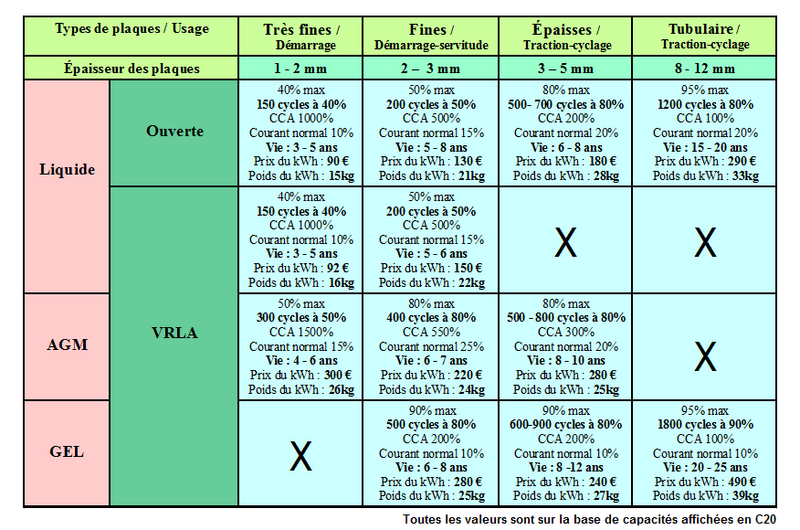
Étape 4 - Different Types of Batteries for Different Uses
There are several types of and technologies for lead batteries, each adapted to a particular use, environment and constraints. Understanding the differences is essential to choosing and maintaining your battery correctly. This part summarizes the main categories of lead acid batteries and their characteristics.
Batteries according to their use :
- Starter battery:
A starter battery is intended to provide high current for a very short time. It is designed to start an engine (for example a vehicle or a generator). Starter batteries are sometimes called "car battery", "truck battery" or "thin plate battery".
View inside a starter battery .
- Traction battery
The name of these batteries comes from their first use: powering the motor of electric vehicles such as forklifts. They are generally equipped with "thick or tubular plates" which allows them to withstand fairly deep discharges and have a long lifespan. They are well suited for use in solar photovoltaics.
The batteries OPzS (liquid electrolyte) and OPzV (gel electrolyte) have almost the same characteristics as traction batteries.
- Stationary battery
These batteries are used in emergency power supplies, in particular for computer or telecommunication systems. They are designed so as to be constantly recharged and to be discharged only infrequently.
- Solar battery / slow discharge
These batteries are intended for use in photovoltaic solar installations. They are designed to withstand a high number of cycles (since they will be discharged every night and recharged every morning), and their depth of discharge is generally good but can vary greatly from one model to another. Service batteries have almost the same characteristics as solar batteries.
View the inside a slow cycle/solar battery
Batteries according to their technology / electrolyte
- Open battery
An open battery is a battery with liquid electrolyte equipped with plugs allowing to fill it. Open batteries are not watertight: the liquid inside evaporates little by little, so it is necessary to check its level regularly and top it up if necessary with distilled water.
| Advantages | Disadvantages |
|---|---|
| Repairable | Maintenance required |
| Delivers current in cold temperatures ( Their CCA rating indicates the battery has sufficient power to crank an engine in very cold temperatures) | Risk of non-homogeneity of the electrolyte if little used = premature aging |
| Withstands overloads and overheating (one can add liquid if it evaporates) | Release of hydrogen and, therefore, risk of explosion if environment not ventilated |
| Low cost | Not conducive to cold, risk of electrolyte freezing |
| Strong self-discharge (10-12% per month) if not used regularly. | |
| Leaks possible if there is tilting or shaking/vibrations |
- Sealed, leak-proof liquid batteries
A sealed battery is a liquid electrolyte battery equipped with a system to prevent the evaporation of the water contained in the electrolyte, by gas recombination. These batteries do not require maintenance, and are often called VRLA for "Valve Regulated Lead-Acid batteries".
| Advantages | Disadvantages |
|---|---|
| Reduces explosive gas production, water loss and leakage. | Does not allow maintenance or control. |
| Requires less maintenance. | Imposes a perfectly regulated load according to the temperature to avoid gas losses by excessive pressure. |
- AGM batteries
AGM batteries are a type of sealed/VRLA battery, in which the electrolyte is a liquid but it is held in place in a fiberglass blotter, and hence its name: Absorbed Glass Material.
View the inside of an AGM battery
| Advantages | Disadvantages |
|---|---|
| Maintenance-free with minimal release of gas | They do not perform well in hot conditions (loss of electrolyte in the form of gas at higher temperatures) A temperature above 49°C (120°F) is very dangerous for the battery life. |
| They maintain the electrolyte homogeneity well. | They are sensitive to overcharging and high voltages (loss of electrolyte in the form of gas) |
| Withstand colder temperatures well because of their homogeneous electrolyte (Since the electrolyte is held in the glass mat separators, it won't expand when frozen like it will in a flooded battery) | They have limited shelf-life (as the acid concentration inside is higher than in others, which leads to faster battery degradation). |
| Allows high peak currents (CCA) to pass | |
| Shock-resistant (Vibration-resistant) (Because of the fibre glass mats are woven tightly and the plates are packed tightly, making them immune to vibrations) | |
| Low self-discharge (1-3% per month) |
- Gel batteries
Gel batteries are a type of sealed battery / VRLA. In a gel battery, the electrolyte is gelled by adding silicate.
View the inside of a gel battery
| Advantages | Disadvantages |
|---|---|
| Maintains the homogeneity of the electrolyte perfectly. | Limited peak current. |
| Low self-discharge (1-3% per month). | Slow charging and discharging. (charge current limited to 5-10% of capacity). |
| Cannot withstand high temperatures (loss of electrolyte in the form of gas - permanent effect). | |
| Longer life span/shelf life. | Sensitive to overload (loss of electrolyte in the form of gas). |
| High cost |
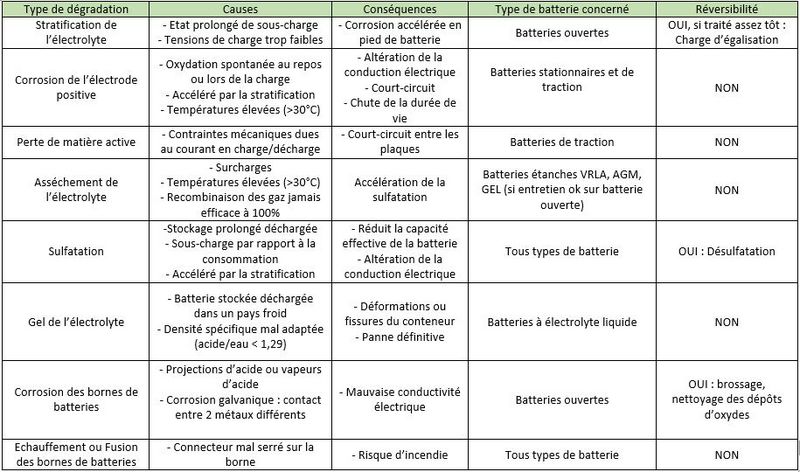
Étape 5 - Mechanisms of degradation of Lead Acid Batteries
- Stratification of the electrolyte: In a wet electrolyte battery, if the electrolyte is not agitated, the sulfuric acid will flow down the trays./plates. Thus, the density of the electrolyte will slowly increase at the bottom of the batteries, while it will decrease at the top of the batteries. This stratification of the acid will result in non-homogeneous electrode discharge with accelerated corrosion at the bottom of the battery.
- Corrosion of the positive electrodes: The positive electrodes are sensitive to corrosion which occurs when not in use, but especially during charging, when the lead in the grid is transformed into lead oxide, which is not very conductive. If there is too much corrosion, the active materials gradually sink to the bottom of the accumulators, and electrodes disintegrate. The capacity of the battery decreases and the internal resistance increases until the battery becomes unusable.
- Loss of active material: During charge and discharge cycles, the positive and negative plates undergo strong mechanical stresses (high currents, induced magnetic fields). The plates gradually disintegrate and the active material accumulates at the bottom of the battery. This "mud" can cause short circuits between two plates.
- Drying of the electrolyte: Naturally, the water contained in the electrolyte evaporates a little. Valve regulated lead acid (VRLA) batteries promote its recondensation, which reduces the need for additional distilled water (unlike open batteries). But, once the battery is charged, a supply of current initiates the electrolysis of the water with the formation of oxygen and hydrogen gas. In a VRLA battery, beyond a certain pressure, safety valves let the water escape permanently. This is problematic because topping up with distilled water is not possible.
- Sulfation: During discharge, crystals of lead sulfate (PbSO4) form on the positive and negative electrodes. If the battery remains discharged for a long time, these lead sulphate crystals grow and harden irreversibly. This reduces the conductivity of the electrodes, causes the battery to lose capacity and can cause short circuits.
Freezing of Electrolyte: When a battery is discharged, the electrolyte is mostly water. At low temperatures, it can freeze and irreparably damage the battery.
Corrosion of the battery terminals : Following acid splashes, acid vapours, or simply galvanic corrosion (two metals brought into contact), lead oxide deposits may form on the battery terminals. This can cause electrical conductivity problems.
- Fusion of the battery terminals: If the connector is loose on the terminal, the electrical contact resistance will increase. When a high current passes, the terminals can melt by the Joule effect (the conversion of electric energy into heat energy by resistance in a circuit). This can lead to fires.
Étape 6 - Summary of good practices to adopt with lead acid batteries
Equipment: Choose your battery carefully according to the intended use.
Never mix new and used batteries.
Never mix batteries of different technologies.
Correctly and solidly install the wiring of your battery bank to avoid fires.
Regularly check the connectors if they are subject to vibrations.
- Detection and Prevention of Deep Discharge: Battery life is directly related to DoD or depth of discharge. It is, therefore, very important to prevent any discharge over 50% !
- How to know the level of charge (SoC)?
- Simply measuring the voltage does not suffice as several factors affect the battery voltage.
- A battery monitor must be used. It calculates not only the voltage but also the charge and discharge currents, which allows the state of charge to be calculated in real time.
- How to avoid deep discharges?
- The idea is to control the level of charge (SoC) and to disconnect the consumption loads as soon as they fall below a certain level.
- Use a battery protector/Battery Protect or a configurable solar charge regulator, for direct current (DC) equipment.
- Use the dry contact relay (voltage-free relay) of your battery monitor if it is equipped with one.
- Set the low battery voltage threshold on your inverter for alternating current (AC) equipment (read the instructions carefully).
- How to know the level of charge (SoC)?
- Pay attention to the temperature: : This factor has a very important influence on the life of the batteries. It is very important to keep the batteries at “cool” temperatures, around 20°C.
- Technical roome : Always choose the coolest room or location. Never leave batteries exposed to direct sunlight. If the place is still too hot, one should consider cooling ventilation of the room or the battery container.
- Aeration and ventilation : Always keep space between the batteries (about 5 cm), do not put them against each other. If the batteries are inside a battery box or in a cabinet, there must be air circulation.
- Temperature compensation: When the temperature exceeds 30°C or is lower than 10°C for a long time, it is necessary to change the charging voltage.
Battery not in use – Self-discharge: When a battery is not in use, it slowly discharges. This phenomenon depends on the type of battery and the temperature. o An unused open battery must be recharged every four months at room temperature (between 10-25°C). o An unused open battery must be kept permanently charged in temperatures below 0°C. o Sealed batteries can be left for up to 6 to 8 months without recharging at ambient temperature. o When a system containing batteries (RV, car, etc.) is not used for a long period, disconnect the batteries to avoid leakage currents.
- Correct charging voltages: Never recharge the batteries with a voltage higher than that recommended in the manufacturer's data sheet. Use a charger with at least 3 charge stages (Bulk, Absorption, Float).
- Correct charge/discharge current: It is recommended never to charge or recharge lead batteries at more than 0.2C, i.e. 20% of the capacity of the battery bank (ex: 20A for a battery bank of 100Ah).
Étape 7 - Desulphation/Regeneration of lead acid batteries
During discharge, lead sulphate (PbSO4) forms on the positive and negative electrodes. If the battery remains discharged, this lead sulphate crystallizes and hardens. Once crystallized, it can no longer turn into sulfuric acid when charging the battery. This causes the battery capacity to drop: "it no longer holds a charge" it is a weak battery/it is a dead battery.
Battery regeneration is a process of sending high-intensity electrical pulses (300-400A) at a given frequency, based on the battery's resonant frequency. This is calculated automatically by the machine and evolves over time. These impulses break down the crystalline layer formed by the amorphous lead sulphate deposited on the electrodes and convert it back to sulphuric acid. The plates are reconstituted and the battery returns to its original condition.
Success rate: Since sulphation is not the only phenomenon underlying battery degradation, not all of them can be regenerated by desulphation.
- For batteries with tubular electrodes, the success rate is around 90% (source: BeEnergy)
- For starter batteries, the success rate is around 30%. (source: BeEnergy)
Duration of the process: This process can last from a few hours for a starter battery to several days for traction batteries.
Research to follow (For Further Reading)
Notes et références
Document by Guénolé Conrad with the help of Loup Girier, Wiam Razi, Elliot Harant and Pascal Criquioche within the framework of the Scholar Grid project. A project initiated by the Schneider Electric Foundation with the technical support of Energie Sans Frontières, Atelier 21 and the Low-tech Lab
- Document Victron Energy, Translated from: “Optimizing the life of lead batteries - Lesson V02 Bis.docx” by Margriet Leeftink, by Jacques Noël”
- Summary on the Installation of lead batteries: Website: Batterie-solaire.com
- Summary on the Maintenance of lead batteries: Website: Batterie-solaire.com
- Report "State of the Art of Desulphation Technologies for Lead-Acid Accumulators" by ADEME - 2011
- Video "Liquid battery, AGM, GEL, what to choose?" from the Youtube channel of Guillaume Piton - La Watterie
Published

 Français
Français English
English Deutsch
Deutsch Español
Español Italiano
Italiano Português
Português